The Hard Facts of Sapphires and Rubies
Ask any jeweler to list the gemstones with which they prefer to work, and sapphires and rubies would undoubtedly rank near the top. With a hardness of 9 on the Mohs scale, these popular varieties of corundum have long been considered nearly impossible to damage, and they can easily withstand the melting point of even high karat gold solders. Not surprisingly, many jewelers feel a comforting sense of security when faced with a ruby- or sapphire-set piece. Which may be a problem.
14 Minute Read
Ask any jeweler to list the gemstones with which they prefer to work, and sapphires and rubies would undoubtedly rank near the top. With a hardness of 9 on the Mohs scale, these popular varieties of corundum have long been considered nearly impossible to damage, and they can easily withstand the melting point of even high karat gold solders (about 1,6400F to 1,7400F). Not surprisingly, many jewelers feel a comforting sense of security when faced with a ruby- or sapphire-set piece. Which may be a problem.
Given the gem's reputation for toughness, jewelers sometimes take the strength of a sapphire or ruby for granted. It's widely accepted, for example, that corundum is one of the few stones other than diamond able to withstand the heat used to retip a prong or perform stone-in-place casting. And that's true; many rubies and sapphires undergo these processes with little or no noticeable effect. However, if a piece of jewelry is subjected to a torch and then rapidly quenched in pickle - a routine followed by hundreds of bench jewelers every day - corundum can crack like an ice cube, and so too can its aura of invincibility.
Nor is corundum able to withstand all temperatures. Jewelers must take care not to overheat corundum - or overlook a possible glass filling, or work near it with the wrong abrasive: Any of these steps could lead to embarrassing and costly mishaps. In our more than 20 years as jewelers and appraisers, we've seen such incidents happen all too frequently. Fortunately, they can be minimized with just a few simple precautions. By taking even a small amount of care, jewelers can prevent a broken or damaged stone and maintain a sparkling reputation.
Before the Bench
Prior to picking up a torch or a file, jewelers should first learn a little bit about corundum and its characteristics. As we've noted, corundum offers a comfortable "safety zone" for most manufacturing operations. Part of the reason for this is that it lacks the delicate cleavage of some other popular gemstones. However, this doesn't mean it can't split apart; another of the stone's characteristics can offset this advantage and lead to what is commonly referred to as "parting."
Parting results from "lamellar twinning," a process in which two crystals grow together in the same plane. This twinning occurs quite often in sapphires and rubies, and it unfortunately becomes as much of a weak point as cleavage planes do in other gemstones: If enough force is applied, the twinned area may de-laminate or part. This problem occurs mostly in star rubies and star sapphires, since the cabochon cut tends to expose thin areas of lamellar twinning planes at the top or rounded bottom of the stone.
However, parting can happen to corundum of any type, regardless of cut. To minimize this risk, jewelers should be especially careful when faced with jobs that may require excessive pressure, such as setting a heavy bezel. In such instances, they should take care to anneal the metal and to carefully cut the seat to match the shape of the stone.
Jewelers should also examine how the stone has been shaped. As with the star sapphires and rubies, the cut can sometimes pose more difficulty than the material itself. We've all seen rubies and sapphires that are fashioned with pavilions shaped like bathtubs. These rotund shapes can make setting difficult, since more metal must be ground out of the prongs to accommodate the bulging pavilion. Consequently, jewelers will often over-burr the prongs, making them thinner and more likely to break or otherwise fail. Using heavyweight prongs and slightly larger heads can help alleviate this problem; as a rule of thumb, the additional burring should take away no more than 50 percent of the prong's thickness.
Of course, some cuts are too unwieldy to allow any type of neat setting. Most sapphires and rubies will have a strange, bulky shape when they're sold. In the past, many jewelers believed they were cut this way simply to meet the laws of countries that forbid rough from being exported, or to maximize weight. However, there's also another possible reason: These cuts may conform to the zoning of color in the stone.
In sapphire and ruby, concentrations of color don't always appear uniformly from one stone to the next. Consequently, a jeweler who recuts a gem to fit a specific setting might do more harm than good: If some of those concentrations rest near the surface, the modification might actually remove color and reduce the stone's value. While this isn't usually a problem, jewelers should think twice before indiscriminately recutting a sapphire or ruby. One simple test that could help is to place the gem, table down, in a glass of water; the water's refraction will emphasize the color saturation and provide a guide for "safe" cutting.
If You Can't Stand the Heat…
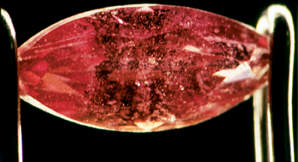
| A ruby marquise prior to heating with a jeweler's torch (left) and after excessive direct heat has been applied. Note the two small white crystals in the center of the stone have "exploded," causing the stone to fracture. | |
| The ruby (left) and sapphire (right) pictured above both exhibit the results of excessive heat in combination with firecoat, in which the firecoat has formed circular areas of film on each stone's surface. The sapphire also has a metallic residue on the film, the result of scraping with a steel probe. | |
Obviously, the cut and characteristics of corundum can influence a jeweler's success. However, most inherent limitations or obstacles can be overcome by a careful approach to the job at hand-which can sometimes be much harder than it sounds. As we noted earlier, corundum's deserved reputation for hardness can sometimes lull jewelers into a false sense of security. Consequently, they may begin ignoring potential problems, such as the effects of heat.
While sapphires and rubies can be damaged in various ways during the repair or creation of jewelry, heat is the most common culprit. It's not that corundum is sensitive to heat, but that overconfident jewelers can sometimes mishandle the heat's application.
Part of this can be the result of routine. For many jewelers, the practice of "heat then quench" becomes reflexive after a while. However, reflex in this instance can pose a problem. Most jewelers will usually heat a piece with the corundum still set-and whether performing torch work or casting stones in place, jewelers who heat and then rapidly quench a sapphire or ruby run a great risk. We've been called on several times to examine corundum with one large, visible crack and several spider-web-like fissures. When asked for an explanation, we've had to explain that these once-beautiful stones had been damaged by thermal shock-an experience similar to that of dropping an ice cube in water and hearing it crack due to the sudden temperature change.
We've seen corundum quenched at the bench with no ill effects many times, but we've also seen it crack often enough to know that the practice is not advisable. Instead, allow corundum-set jewelry to cool before pickling; quenching in this way is perfectly acceptable, and has not been known to cause damage. In fact, as a matter of course, jewelers should remove or cover the pickle pot when working with sapphires or rubies; old habits die hard, and reflexes can always reassert themselves.
Not all problems can be blamed on routine, though. For example, yellow sapphires can lose color when heated (although sometimes that color may return). To be safe, jewelers should take any yellow sapphire out of its setting before beginning a repair. If that isn't possible, coat the entire stone with a heat-retardant gel. They may also submerge the mounted stone in water, depending on where the repair is needed.
Problems can also result simply from bad techniques. A bench jeweler once complained to us that whenever she used firecoat on a ruby or sapphire during retipping, an unremovable film formed on the stone's surface. As we thought back, we recalled seeing several stones over the past few years with the same type of problem. Although the film could sometimes be scratched away with a steel probe, it was otherwise nearly impossible to remove, short of repolishing.
Examining a prong setting with a film-coated sapphire, we noticed that the retips themselves were pitted as though they had been overheated. A little experimentation seemed in order, so we retipped rubies and sapphires with several types of gas torches-propane/oxygen, natural gas/oxygen, and acetylene/oxygen. We also tried a water welder with a self-fluxing feature, in which the unit actually breaks down distilled water into hydrogen and oxygen, then sends the mixed gas through a fluxing solution of methyl alcohol and boric acid. With each setup we intentionally overheated the stone.
Our findings: When the prongs overheated to the point of pitting, the firecoat did indeed form a film that could not be removed. (Our theory is that the heat actually cooks the borax into the surface of the stone, but we didn't have the electron microscope to verify this.) We also noted that the pinpoint flame and self-fluxing action of some water welders made it easier to avoid overheating, although the flame burned very hot-which could cause a problem in unskilled hands.
Ultimately, though, the key to avoiding an ugly film does not lie in changing torches, but in learning good technique. For example, jewelers shouldn't use excessive borax in the firecoat, since the borax may "clump" on the stone. They should also focus the flame on the prong tip, so as not to heat the stone excessively. Such practices-as well as a better understanding of corundum's limitations-will lead to fewer problems and more durable, attractive retips.
Treating Corundum Well
When working with sapphires and rubies, jewelers also must be aware of the various treatments used to enhance corundum. While not all such treatments will pose a threat, some may-and the smart jeweler will be on the lookout for them.
First, nearly all natural rubies and sapphires are heat treated. While the devices used to accomplish this may vary-they can range from crude metal buckets to sophisticated computer-controlled furnaces-the purpose remains the same: to improve a stone's color or clarity though heating and subsequent cooling in a controlled environment. The specific temperature, environment (either oxygen rich or oxygen depleted), and length of treatment all depend on which of those goals is being sought, as well as on the condition of the original stones.
Heat treating is usually a benign method of improving corundum's appearance, but it will cause some structural changes. During treatment, voids and other inclusions in the stones will expand and contract at different rates from the surrounding corundum, and this can often cause cracks in the stones. Consequently, many heat-treated sapphires and rubies have "exploded" crystals. These crystals are usually contained within the stones and are non-damaging. (If material is damaged, it is generally either recut-if salvageable-or discarded.) Moreover, the heat from a torch rarely will cause any further cracking
Actually, jewelers will have the most problems with corundum that has not been heat treated. The unenhanced rubies and sapphires are usually the rarest and most costly; many of these have documentation from gemological laboratories attesting to their untreated condition, and buyers will pay a premium for them. But because they have not been treated, they may have inclusions that could "explode" when heated during a repair. This could lead to severe loss in value for the stone-and a possible lawsuit for the jeweler.
The best way to avoid accidental damage to these untreated stones is to learn how to recognize the types of inclusions found in them, and then avoid any torchwork. When viewed through a microscope, untreated stones might display "negative crystals" (i.e., voids that resemble a solid crystal), undissolved rutile needles, or crystalline deposits of other minerals (similar to negative crystals). If in doubt about whether such inclusions exist, jewelers should take an untreated stone out of the mounting or coat it with a heat-shield before the repair begins. (They may also submerge the mounted stone in water, depending on where the repair is needed.) If none of these options is possible, the owner of the jewelry piece should be fully informed of all risks before any work is performed, and the jeweler should make clear for what he or she will take responsibility. We strongly recommend that every jeweler have a written policy that they discuss point by point with the client, and that they have the client sign it.
Diffusion treatment is another method that adds color to corundum-usually sapphire, although diffusion-treated ruby can be found on the market in small amounts. The treatment consists of packing colorless or light-hued faceted sapphires or rubies in powdered chemicals that naturally cause the color of corundum; a blue sapphire, for example, might be packed in titanium oxide. These chemicals are heated at temperatures of about 2,0000C, until they melt into the stone. The surface is then repolished to eliminate the ripples caused by the high temperatures, until only a thin crust of color is left.
Diffusion-treated stones generally remain stable when exposed to heat. However, any ruby or sapphire exposed to this treatment will now have a surface color different from that of the interior. This means that any chips or nicks caused during setting or finishing will be highly noticeable, and jewelers won't be able to simply polish them out. For this reason, every sapphire or ruby should be illuminated from behind with diffused light, and the jeweler should look for the telltale areas of darker color along facet junctions. This will allow the jeweler not only to take extra precautions, but also to provide full disclosure to the client.
Then there's the matter of fracture filling. In recent years, the number of sapphires and rubies with glass-filled cavities and fractures has surged. While some analysts debate as to whether this is an intentional practice or the result of packing the gems in borax during heat treatment, everyone agrees that any gem with a glass filling must be handled extremely carefully. Since the melting point of glass is typically lower than that of solder used in prong work, heating such stones will result in the filler oozing out of fractures or disappearing from cavities.
The best way to avoid explaining why a ruby or sapphire now looks like Swiss cheese is to learn how to recognize the treatment. To spot a glass-filled cavity, examine the stone under magnification, tilting it back and forth to catch reflected light. Glass is softer than corundum and thus does not take as high a polish. The difference in the quality of the luster will be readily apparent.
Glass-filled fractures may also show gas bubbles trapped in the glass. Once a glass-filled stone is detected, it can then be removed from its setting before any work is done, or be protected with water or a heat-retardant product. If the presence of glass cannot be ruled out for certain, treat a stone as though it were filled.
The Unkindest Cut
As we've noted, corundum is definitely hard. Nonetheless, it is not impregnable-as anyone who carelessly uses abrasives and files will discover. For this reason, jewelers who find themselves in the home stretch of a project can't become overconfident: Sapphires and rubies stand just a good chance of being damaged during the finishing process as they do at any other time.
Most often such damage is the result of finishing prongs with either files or cup burrs. Since these tools are tough enough (and usually used with enough impact) to damage diamonds, they are even more capable of scratching and abrading corundum. Jewelers should always take care not to hit the stone with a file edge or burr. In fact, since one face of the file will always rest against the stone, the teeth on this face should be ground away. This practice greatly reduces the occurrence of abraded facet junctions and scratched stones.
Files and cups burrs aren't the only dangers lurking in the finishing process; diamond abrasives also pose a significant threat. Since diamond is harder than corundum, these abrasives need only a little pressure to cause an ugly surface scratch. Given that more and more diamond-impregnated burrs, discs, and wheels seem to be entering the industry of late, jewelers must always note the type of abrasive that is being used. This is especially true of pre-charged wheels and sanding disks. Keeping a sapphire or ruby safe may be as simple as reading a product label.
However, even if files have been ground smooth and all diamond abrasives have been banished from the bench, jewelers still aren't safe. That's because many of the abrasives used in jewelry manufacturing are actually charged with corundum, and the old adage that "diamond scratches diamond" is just as true for sapphire and ruby. Sanding disks, rubber wheels, stone wheels, separating disks, sanding papers-any of these might contain corundum or silicon carbide (carborundum). Also, be aware that corundum can go by various names: aluminum oxide, emery, even such brand names as Cratex and Adalox. Be sure to always read the abrasive's label; if in doubt, check with the supplier.
Of course, any abrasive applied with an excessive amount of force can damage a ruby or sapphire. In the end, it all comes down to care, and to respecting the limits of the stone. If they keep that in mind, jewelers will be better able to keep their sapphires and rubies-as well as their reputations-intact.
The award-winning Journal is published monthly by MJSA, the trade association for professional jewelry makers, designers, and related suppliers. It offers design ideas, fabrication and production techniques, bench tips, business and marketing insights, and trend and technology updates—the information crucial for business success. “More than other publications, MJSA Journal is oriented toward people like me: those trying to earn a living by designing and making jewelry,” says Jim Binnion of James Binnion Metal Arts.
Click here to read our latest articles
Click here to get a FREE four-month trial subscription.
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.