Anodizing Aluminum
7 Minute Read
While I was in graduate school at San Diego State University I became interested in the anodizing process. I had been working with aluminum in jewelry for a while when Arline Fisch brought back some basic anodizing aluminum information from Bezalel Academy in Jerusalem. I put together a primitive anodizing set-up, which eventually lead to my doing my M.A. thesis in 1981 on the anodizing aluminum process and its applications for jewelry.
I moved to New York City that fall and was offered a teaching position at Parsons/New School. There I met technical consultant Thomas Pignatelli, who helped us put together anodizing equipment. Through him I learned more about the process and became acquainted with the Sandoz Company and their dyes and chemicals, which I now use. The set-up in my studio is based exactly on what is found in industrial situations. This has made the process more efficient and the outcome more predictable.
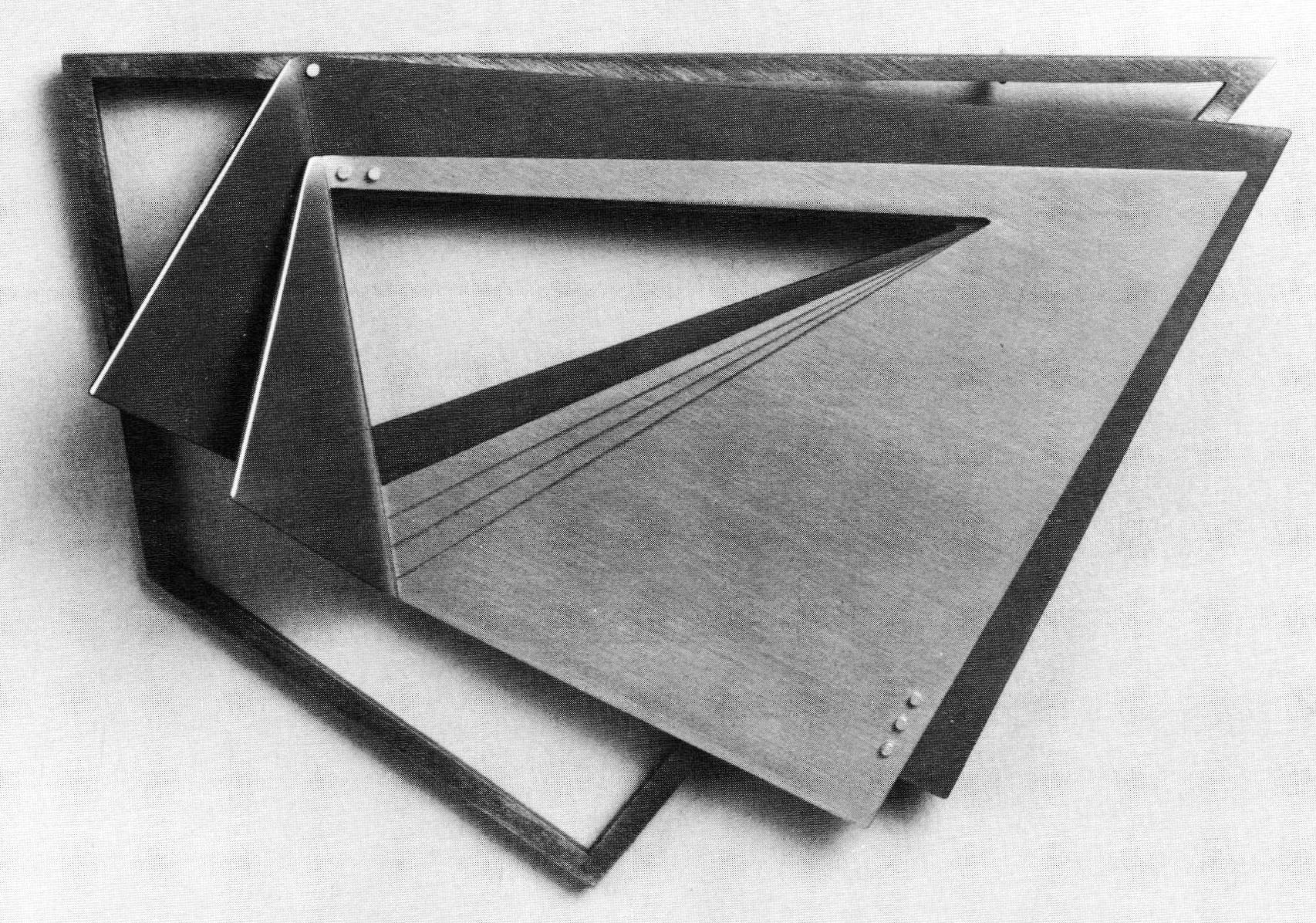
I believe anodized aluminum is an ideal material for jewelry. Aluminum is light in weight, and it can be colored in a wide range of hues through the use of the anodizing process. I find stimulation in the irony of using an industrial material and process for jewelry and combining this with more traditional material, such as gold, silver and stones.
Anodizing is an industrial process that produces a stable oxide film on the surface of aluminum. The work piece is attached to an aluminum hook or wire and then immersed in an acid bath (electrolyte solution), it receives the positive (anodic) current, and as oxygen is released an oxide film containing billions of pores similar to that of a honeycomb structure is produced on the metal surface. These pores will then accept dye. Once sealed, the metal is strengthened and protected from corrosion and is resistant to water, oil, salt, weather and general wear.
For bright, clear colors on relatively pure aluminum the sulfuric acid process is used. A chromic acid process, which produces a less clear, thinner coating and color, is used for less pure aluminum alloys. (See also Jewelry Concepts and Technology by Oppi Untracht, New York: Doubleday, 1982 for more information on the chromic acid process.)
Anodized aluminum has a variety of industrial uses in architecture and building materials, hardware and housewares. Its applications for jewelry are exciting. In addition to the durability of the anodized surface, aluminum is light in weight and the color palette is almost endless. The Sandoz dyes are rated highly in color-fastness and will not fade noticeably for many years. By controlling the variables of time and temperature in the acid bath and the time and temperature of the dyes, multiples of the same color can be easily produced.
The process is a bit more time consuming than that of coloring refractory metals, such as titanium and niobium, but it produces on the aluminum a clear field of color that is much more durable. The color can be solid or shaded through overdyeing. Resists can be used to produce more than one distinct color on a surface, and the dye can also be painted on.
Fumes are created while using the caustic etch and the anodizing bath and the work area must be well-ventilated. (For more information on ventilation, consult Ventilation: A Practical Guide, $7.50, available from The Center for Occupational Hazards, 5 Beekman Street, New York, NY 10038.) As with any electrical set-up, normal precautions should be taken. Never touch the anode (work piece) and cathode (lead) or connecting rods or wires while the rectifier is on. The current generated could be extremely dangerous. Rubber gloves should be worn as insulation against electric shock and also for protection from the acids and lye.
Sulfuric Acid Method Process
Cleaning the metal
- Clean metal using soap on a polished surface or pumice on a sanded surface after completely finishing surface (sanding, polishing, etc.). Metal cannot be worked after anodizing.
- Etch for a short time in Caustic Soda (lye), 4-6 ozs./gallon of water at room temperature for 5-10 minutes.
- Rinse in clean water.
- Immerse in Nitric Dip, 5-10% solution in water, for 2 minutes.
- Rinse in clean water.
Dyeing
- Mix dye in water (deionized or tap), according to Sandoz chart (see Equipment). Most colors require approximately 2gms/liter (¼ oz/gallon). Temperature: Use cool dyes and short anodizing time for pale shades, hot (100-120°F.) dyes and longer anodizing time for darker shades, Time: Hang metal in bath for 5-10 minutes or until desired color is reached, checking color often.
- Rinse in clean water.
Sealing
- Place metal in boiling water containing Sandoz Sealing Salt AS for at least 10 minutes, with ratio of 1 oz./gallon. Make sure your work space is well ventilated, as fumes from this solution can be irritating.
- Rinse thoroughly in warm water and pat dry with a soft, clean cloth.
Notes:
For more than one solid color on a piece of metal, resists may be used. (I use a vinyl synthetic resist, which can be easily peeled off). A resist is applied to a very clean surface before starting the process. For every additional color the piece must go through the process one complete time. To retain the original color resist must be applied to previously dyed surfaces before you begin to dye another part of the piece.
A shaded effect can be achieved by immersing the piece partway into one dye and then immersing the other part in another color, the area where they meet will blend together, similar to watercolor. Dyes may be mixed in the containers, or pieces may be overdyed by immersing in one color, rinsing, then immersing in another color.
Dye may be painted on the surface. The dye should be simmering. The color will be more pale than if it was immersed.
Permanent type felt pens may also be used after anodizing and before immersing in the dye. The piece must be carefully dried before the pen is applied. They act as a resist for the water-based dye while imparting the color of the pen. (Pen colors may not last as long as standard dye colors, but they serve the same purpose.)
Equipment
- Container for Anodizing Solution—polyethelene or stainless steel—size dependent on studio capacity.
- Rectifier—at least 20 volts and 10 amps, to produce DC current.
- Cathodes—lead sheet: approximately equal in surface area to objects to be anodized.
- Thermometers—measuring at least 150°F. (for anodizing bath and dyes).
- Wires and Clips—aluminum wire is good for hooks. Only aluminum or titanium may be in the bath with your piece. Copper may be used for connecting rods (see Diagram A).
- Electric Hotplate for pot of sealer.
- Immersion Heaters for heating dyes.
- Containers for Dyes, Acids and Rinses—polyethelene buckets 3-6 qts. work well.
- Dyes—Sandoz aluminum dyes.
- Sealer—Sandoz Sealing Salt AS (nickel acetate, cobalt acetate, boric acid in equal amounts).
- Chemicals—Caustic Soda (lye)—this substance is hazardous and requires careful handling. Nitric Acid, reagent grade. Sulfuric Acid, reagent grade. Baking Soda.
- Container for sealer—stainless steel pot.
- Aluminum—6061 , 1100, 5052 series are good and readily available as surplus.
- Protective Clothing—rubber gloves, smock or lab coat, goggles.
- Miscellaneous—resists: Kodak photoresist, Asphaltum, or synthetic vinyl resists work well. Felt pens. Paintbrushes.
- Cooling System—adding ice to anodizing bath works temporarily, but a small immersible pump in a bucket of ice water with polyethelene tubes to recirculate the ice water through the anodizing bath works better for longer periods.
Most of the equipment is available through chemical supply companies, hardware stores and electrical equipment companies. Much of it can be obtained through surplus outlets. Buckets may also be obtained from plastics supply companies.
Dyes can be obtained through Met-L-Art in Wilmington, DE, (302) 475-1906. They sell small quantities—2 oz. packages. Additional dye information may be obtained through Sandoz Technical Service Department, (704) 372-0210, 4000 Monroe Rd., Charlotte, NC 28205.
Rectifier: available from I. Shor, Gesswein, Allcraft and all big metalsmithing suppliers. Estimated costs: $150-$200, excluding power supply, which is available for $100-$800.
David Tisdale's work was featured in a solo exhibit at Helen Drutt Gallery, Philadelphia, December 1984—January 1985.
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.