Optimising Gold Alloys for Manufacturing
Standard yellow gold alloys which are based on gold-silver copper can be used for many production processes. They could be called ‘all-purpose’ alloys. However, optimising the alloys for the particular manufacturing process to be employed is possible by use of other alloying additions which can influence relevant properties such as castability and grain size as well as the mechanical ones, for example, strength, hardness and ductility. Only a few elements (e.g. zinc, silicon, iridium, cobalt) have proved to be useful additions for modifying carat gold alloys without detrimentally changing other relevant properties.
20 Minute Read
Standard yellow gold alloys which are based on gold-silver copper can be used for many production processes. They could be called 'all-purpose' alloys. However, optimising gold alloys for the particular manufacturing process to be employed is possible by use of other alloying additions which can influence relevant properties such as castability and grain size as well as the mechanical ones, for example, strength, hardness and ductility.
Only a few elements (e.g. zinc, silicon, iridium, cobalt) have proved to be useful additions for modifying carat gold alloys without detrimentally changing other relevant properties (e.g. colour). The present paper deals only with yellow carat gold, particularly 14 and 18 ct alloys. In the case of very high carat alloys, other modifications must be made to improve the properties. White gold alloys, with their more complicated composition, are a different problem and are not discussed here.
However, all such alloy modifications have to be performed with care. They can confer not only advantages but also disadvantages. Improper use causes defects. The influence, both advantageous and disadvantageous, of the more frequently used additions will be discussed. All elements which are added to standard gold- silver-copper alloys are considered as 'additions', independent of their concentration.
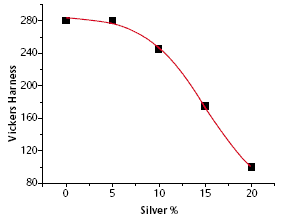
| Figure 1 - Influence of age hardening (at approx. 300°C) on hardness as a function of silver content in an 18 carat yellow gold alloy |
Properties of Carat Yellow Gold Alloys
Alloys based on the gold-silver-copper system can be used for almost any purpose in jewellery fabrication e.g. investment casting, rolling sheet, drawing wire, deep drawing, stamping and chain making. They show an easy machinability. The low-medium carat golds can be age hardened to a remarkable degree, as demonstrated in Figure 1.
Another advantage is that the only constituent which forms oxides is copper (cupric and cuprous oxides). Copper oxides are not very persistent. If formed, they can be easily reduced and oxidation can be avoided by relatively simple methods.
In spite of these advantages, improvement of the alloys is necessary to meet the more demanding requirements of modern production methods and increased demands of customers. Since the late 1970s, many investigations for the development of improved alloys have been performed and the influence of small alloying additions on different types of alloys studied (1-4).
In the following sections, such additions will be roughly classified by effect and purpose:
- Improvement of castability (fluidity, form-filling)
- Prevention of scaling
- Deoxidiser
- Grain refiner
- Strengthener
Difficulties with Additions
Speaking about applications of additions and their advantages and disadvantages, it might be useful to use the terms which are familiar in terms used for medicines, such as:
- Application
- What is the correct dosage?
- Effects and side effects
- Recommendations for application
It should be stated clearly that no effect of additions exists without there being some degree of a side effect which might be detrimental. Also, an often neglected fact is that a positive effect can only be achieved if the correct dosage is used and the range of application is taken into account. Each addition makes the standard alloy somewhat more complicated and modified working conditions may be necessary.
Table 1 - Grain refining in yellow gold alloys
| Type | Field of application | Working mechanism | Examples |
| 1 | Casting | ||
| 1a | High melting temperature, limited solubility in the alloy | iridium, ruthenium, (cobalt) | |
| 1b | High reactivity with oxygen, low solubility, formation of fine dispersed oxides | rare earth (yttrium), boron, barium, (calcium) | |
| 1c | Probably formation of intermetallic compounds | e.g. zirconium/boron cobalt/boron | |
| 2 | Soft annealing | Formation of fine dispersions at annealing temperature | Cobalt, All Type 1 additions can also decrease grain size during soft annealing |
Survey of Additions
In the following, an attempt is made to classify additions which are mentioned in literature. However, this is somewhat arbitrary. Some additions can be placed in more than one class. Last but not least, not all the additions are really used in contemporary practice. The most frequently used additions are discussed later in more detail.
Improved castability (fluidity, form-filling) and prevention of scaling Most of the attempts made to improve alloys concern the casting properties. Investment (or Lost Wax) casting is nowadays the most important fabrication method for gold jewellery. It is also relatively susceptible to defect formation. There is a strong desire to improve the casting procedure and the quality of castings. One possibility for achieving this aim is the improvement of the properties of the alloys used in casting.
Castability and (improved) form-filling are almost synonymous terms. They are most influenced by two frequently used additions: zinc and silicon. It is not quite clear which property leads to improved form-filling. Most likely, it is the reduced interfacial tension. Both elements, as alloying additions, also reduce the formation of the dark porous copper oxide layer during the cooling period. The castings have a brighter surface as a result.
Deoxidiser
There is a considerable confusion about the use of deoxidisers. Principally, yellow gold alloys based on gold-silver-copper should not need deoxidising if melted and cast under proper conditions. Copper oxide, the only oxide formed in this type of alloy, can be easily removed with slightly reducing conditions which can be achieved by use of a protective atmosphere, graphite crucible, or covering the melt with charcoal.
Of course, additions like zinc or silicon will reduce copper oxide with the formation of their much more stable oxides. However, these oxides can be even more detrimental than copper oxide. Especially in the case of zinc oxide, once formed, the oxide is difficult to remove from the melt. So, strictly, it is wrong to call these elements deoxidisers.
Phosphorous and, perhaps, boron act as a real deoxidiser. However, their effect is only beneficial if added in just the right concentration for removing oxygen (reducing the copper oxides). Any surplus phosphorous can be seriously detrimental, causing alloy embrittlement. In practice, it is almost impossible to find the correct concentration. Therefore, the use of such deoxidisers cannot be recommended.
Grain Refiner
One of the disadvantages of yellow or red gold alloys is their relatively large grain (crystal) size and the pronounced dendritic structure in the as cast state. Deformed (cold worked) and soft annealed material would be improved if the grain size is reduced. Large grains are responsible for the orange peel surface effect, leading to increased polishing work, as well as lower ductility, for example. Thus grain refining is particularly desirable for wrought fabrication of jewellery and is important for investment cast jewellery.
The idea of grain refining is not very new. In the early 1970s, attempts were made to apply ruthenium additions as a grain refiner for castings. A later publication gives the results of a comparative study for the grain refining effect of different additions and combinations of additions (5). The following additions and combinations were among those tested: iridium, zirconium, cobalt, boron, yttrium, zirconium + boron and cobalt + boron. Barium was also found to be an effective grain refiner (3). In almost all cases, the additions were in the range of 0.005 to 0.05% per weight. Cobalt was added up to 0.5%. More detailed investigations showed that a distinction has to be made for grain refining obtained during casting and grain refining during soft annealing after cold working.
All the aforementioned grain refining additions act in a similar way. They form really fine dispersed nuclei as starting points for the formation of grains (crystals) at solidification (crystallisation) or at soft annealing (recrystallisation). In all cases, small concentrations are effective. In Table 1, the grain refiner additions are classified according to the field of application and the working mechanism. More detailed information is available from literature, for example, references (6) and (7).
'Strengthener'
There is no great demand for additions which increase hardness and tensile strength of 14 and 18 ct alloys, especially considering that the potential provided by age hardening of conventional alloys are not fully used. The situation is different with high carat alloys (21, 22 ct and higher). Investigations have shown that attempts to increase strength with conventional alloying elements are not effective. A solution is the use of systems which provide age hardening effects. The systems gold- zirconium and goldtitanium have been investigated. Gold-titanium alloys ('990′ gold) with 1 to 1.5% titanium proved to be very useful. However, modified working conditions are necessary, which have restricted the general acceptance of these alloys by industry.
A more recent development is the so called 'pure gold' (or 24 carat gold) with increased hardness. A better term for such materials is micro-alloyed gold. Additions of calcium, rare earth and other elements at a very low level of addition significantly increase the hardness and strength. These additions lead to a twophase structure which results in dispersion hardening.
Grain refining itself can increase hardness and tensile strength to a limited extent.
Improvement of Standard Yellow Gold Alloys
In the following the use and the effect of some frequently used additions are treated in more detail.
Zinc
Application
Zinc is by far the most widely used addition to carat golds. In low carat gold ( 8 to 10 ct) and in nickel white gold alloys, zinc is a normal constituent that has been used since the early development of these alloys, typically in the concentration range up to approximately 10%. It influences the colour and improves the castability. However, the alloys are not homogeneous. Segregation on grain boundaries favour, for example, intergranular corrosion and/ or stress corrosion in 8 to 10 ct alloys. The effect of zinc additions on the quality of 14 and 18 ct yellow gold investment castings has been well known for many years (8). High concentrations of zinc are used in low-medium carat wrought alloys to improve alloy workability, through contraction of the two phase field, and in carat gold solders where it reduces the melting range.
Zinc can be alloyed into gold and gold jewellery alloys to a limited extent without changing the microstructure. The specific amount of zinc which can be tolerated depends on fineness (gold content) and the silver:copper ratio. Gold itself can dissolve approximately 3% Zn (by mass) without any change in microstructure. Higher concentrations cause the formation of second phases including intermetallics. A detrimental influence on properties can be expected. In 14 and 18 ct alloys, higher concentrations of zinc are possible due to the solubility of zinc in silver and copper.
In this paper, zinc is considered only in the context of a small addition for improving of yellow gold alloys. Its main effect lays in casting properties in investment (lost wax) casting.
Dosage
The best effect will be obtained with a maximum of 3% zinc. Normally 2% will be sufficient for good form-filling and a bright cast surface. Higher concentrations have been used at times. They change the colour and reduce the melting range. However, unwanted side effects have to be taken into account too.
Effects on the investment casting of yellow gold (9)
Zinc as an addition in casting alloys has several very beneficial effects, if used in the right concentration. The effect can be summarised as follows:
- Increased the form-filling
- Reduced surface roughness
- Reduced reaction with investment
- Brighter surface in the as-cast state
Increased form-filling
In Figure 2, the influence of zinc additions up 2% on the form filling of 14 and 18 ct yellow gold is shown. All the tests were performed with a standard test grid under constant conditions. The beneficial effect is remarkable.
| Figure 2 - Influence of zinc on form-filling of a test grid in investment cast 14 and 18 carat yellow golds |
Reduced surface roughness
The surface of investment cast items comes out much smoother if the alloy contains 1 to 2% Zn. The effect is more pronounced with heavy parts. A reduction of roughness to approximately one third can be achieved.
The reason for this is that both increased form-filling and reduced roughness can be related to the effect of zinc on the interfacial tension. Measurements of interfacial tension showed that reduction to approximately one tenth of the value of a zinc-free alloy might be possible. (However, it has to be said that the results were not very reliable, probably due to variations in casting atmosphere). A reduced interfacial tension improves the wetting of the investment with the melt, and capillary forces are reduced. The melt can fill thin cavities more easily and reproduce the smooth surface of the pattern (avoiding a dendritic structure on the surface).
Brighter surface in the as cast state
Zinc has a stronger affinity to oxygen than gold, silver and copper. During the cooling period of a casting, a thin and relatively dense layer of colourless zinc oxide is formed on the surface of the cast item. It replaces the thick black, porous layer of copper oxide, Figure 3, normally seen on zinc-free carat golds. The castings have a bright yellow appearance. Pickling removes the zinc oxide layer easily without any discolouration of the surface.
Reduced reaction with investment
Small zinc additions have been proven to reduce the reaction with the investment and, in this way, reduce also gas porosity. The reason for this is not quite clear but, probably, the formation of a dense layer of zinc oxide at the surface of the solidifying melt prevents the interaction of the melt with the investment.
Side effects
Zinc is an addition with relatively few detrimental side effects, especially if the concentration is kept low. The main disadvantages are:
A) The formation of stable zinc oxide
The oxide frequently forms thin film or membrane-like inclusions in the melt which do not easily separate from the melt. Under some conditions, nests of inclusions are formed as demonstrated in Figure 4. The danger of producing these defects increases rapidly:
- a) with increasing zinc concentration,
- b) when melting and casting under oxidising conditions,
- c) when using polluted material as the charge for melting.
Factors a) and c) are probably the main reasons for the defects.
B) The high vapour pressure of zinc
Pure zinc boils at 907°C, that is, the vapour pressure reaches atmospheric pressure. Added as a small addition in gold alloys, the partial vapour pressure of zinc is significantly reduced. To a very rough approximation, the vapour pressure of zinc is reduced to 1/20 the value of pure zinc in an 18 ct alloy with 2% Zn, and to 1/10 the value in a similar alloy with 5% Zn. On the other hand, the vapour pressure increases strongly (exponentially) with temperature. An increase in temperature of 200 K (°C) more than the boiling point of pure zinc increases the vapour pressure by factor of 5.
C) Increased reaction with the investment
This happens if too high a concentration of zinc is used. If the optimum zinc addition is exceeded, the reaction with the investment increases again, leading to gas porosity and surface defects.
Recommendations for application
In general, additions of zinc to jewellery alloys based on gold silver- copper can be recommended. The restrictions mentioned above, especially concerning the concentration range, should be taken into account. Melting of zinc-containing alloys may result in zinc loss by evaporation of zinc. The extent depends on concentration and temperature. The danger of loss is lowered with low zinc concentration, use of a reduced pressure atmosphere (instead a high vacuum) and a reduced melt superheat (excess melt temperature over liquidus temperature of alloy).
| Figure 3 - Influence of zinc on surface quality of as-cast yellow gold Left: Zinc free Right: 2% zinc |
| Figure 4 - Surface defects caused by inclusions of zinc oxide |
Silicon
Application
Silicon shows no significant solubility in pure gold and pure silver. In both cases, a low melting eutectic (gold-silicon: 363°C) is formed even if the concentration of silicon is very low and this eutectic occurs at grain boundary regions. This means that at temperatures below the solidus temperature, some melting of the alloy occurs locally on grain boundaries. This segregation at grain boundaries make the alloy brittle.
Copper has a higher solubility for silicon (approximately 5%). Therefore, yellow carat gold (Au-Ag-Cu) can tolerate a small amount of silicon without producing silicon segregation at boundaries. Thus, silicon can be used for improving the casting properties. Silicon is often called a deoxidiser because of its high affinity for oxygen, with the formation of a very stable oxide. Several publications deal with the use of silicon to improve casting properties of yellow gold (10-13).
| Figure 5 - Brittle fracture of a ring with silicon additions |
Dosage
The maximum concentration of silicon that can be used without producing embrittlement depends strongly on the copper content and, therefore, also by definition on gold fineness and silver content. In a relatively copper-rich 18 ct alloy with 4.5% Ag, 18% Cu and 2.5% Zn, the maximum concentration of silicon is approximately 0.05% by weight. An addition of 0.1% Si causes complete embrittlement of the alloy. In contrast, 14 ct yellow alloys may contain approximately 0.04 to 0.08% silicon and 10 ct alloys can tolerate an even higher concentration of silicon due to their higher copper contents. Additions of 0.15% to 0.35% Si were found to have no detrimental effect.
| Figure 6 - Brittle fracture due to silicon additions (detail of Figure 5) |
Effects on the investment casting of yellow gold
Silicon as a small addition in casting alloys has (like zinc) some
beneficial effects in casting alloys. The effects are:
- Increased the form-filling
- Reduced reaction with investment
- Brighter the surface in the as-cast state
- Reduced surface roughness
The reason is probably similar to that given for zinc additions: A reduced interfacial tension is responsible for the increase of 'fluidity' and the formation of a dense, colourless oxide layer results in the brighter surface in the as-cast state. Thus, the melt can more easily fill thin cavaties and reproduce the smooth surface of the pattern. As with zinc, the reaction with investment might be reduced too.
Side effects
Silicon, as an addition to carat golds, has two serious side effects:
- Embrittlement of the alloys by formation of low melting brittle phases segregated on grain boundaries.
- Production of a very large grain size, further increasing the danger of fracture.
As has been already discussed, silicon is only soluble in the alloys to a very limited extent. Any surplus silicon in excess of this solubility causes brittle fracture, as seen in Figures 5 and 6. This 'disease' is the origin of numerous rejects in jewellery manufacture.
Silicon is also one of the most effective grain coarseners. The coarse (large) grain size favours not only fracture, especially if small amounts of a low melting silicon compound are present, but acts also detrimentally to the polishing behaviour. A grain refining addition is inevitably necessary if silicon additions are to be used in practice. However, according to McCloskey et al (12), silicon may form silicides with many grain refiners. The grain refining effect is extinguished and hard inclusions are formed. In spite of this result, iridium is sometimes used as a grain refiner in practice.
Recommendations for application
Without doubt, silicon is a very effective addition for increasing 'fluidity' of the melt with consequent improved form-filling and surface quality. It also produces a very bright surface on cast items. Many commercial master alloys and carat golds contain silicon additions. On the other hand, serious detrimental side effects can occur, as described above, which increase the manufacturing reject rate drastically. It should be used only with care in an amount which is well adapted to the composition of the base alloy. Due to the reducing maximum addition with increasing gold content, the use of silicon in high (21, 22 ct) carat golds is not advised.
| Figure 7 - Grain refining effect of 0.0055 iridium in 18 carat yellow gold (50x) | Figure 8 - 18 ct yellow gold without iridium additions (50x) |
Iridium
Application
Iridium and ruthenium are well known as grain refiners over many years. They form fine dispersed particles in the melt in advance of solidification of the bulk alloy. These particles act as nuclei for crystal (grain) formation and growth. The grain size is inversely related to the number of nuclei. The more nuclei formed, the smaller the grain size in the alloy.
Dosage
In 14 and 18 ct gold alloys, a 0.005% iridium addition produces a fine (small) grain size in casting. An addition of 0.01% is more than sufficient in any case. Higher concentrations should be avoided (see side effects).
Effects on the investment casting of yellow gold
The grain refining effect of 0.005% Ir in 18 ct yellow gold is demonstrated in Figures 7 and 8. Three other effects are connected to grain refining:
- The amount of dendritic segregation from casting is reduced
- Accumulation of large pores and nests of pores is reduced. However, the total amount of porosity is not reduced.
- Mechanical properties are influenced beneficially.
Side effects
Segregation of iridium particles can occur which are responsible for hard spots which affect polishing, Figures 9 and 10. There are three reasons for such defects:
- Too high an iridium concentration
- Using a wrong master alloy
- Melting temperature too low or/and melting time too short.
| Figure 9 - A nest of iridium particles on the surface of a 21 carat gold alloy | Figure 10 - Iridium inclusions in 14 carat yellow gold (micro-section, 125x) |
Recommendations for application
Iridium is a very useful grain refiner. The concentration should be limited to 0.01%. Alloying is somewhat difficult. A good homogeneous copper master alloy is necessary. Dissolving of iridium in the molten gold alloy takes some time and the melt temperature should not be too low. Note: Ruthenium has a similar effect; however, achieving a homogeneous distribution in the melt is more difficult.
Cobalt
Application
It is often stated that cobalt is a grain refiner in the solidification of casting. Higher concentrations can sometimes give an extremely fine grained structure, although detrimental side effects (see later) will occur. Therefore, cobalt cannot be recommended as a grain refiner for castings. However, cobalt is an effective grain refiner during soft annealing (recrystallisation) of cold worked material (15). Detrimental side effects are unlikely to occur if used in the correct concentration.
Dosage for the use of cobalt in soft annealing
The critical point for use of cobalt as a grain refiner in soft annealing is to provide fine dispersions of nuclei at the annealing temperature without producing coarse particles during initial solidification. The solubility of cobalt in the alloy at solidification must not be exceeded. It is determined, in the first place, by the silver concentration of the alloy.
Depending on silver content (and gold fineness), the useful addition of cobalt ranges from approximately 0.15 to 0.5%. It has to be chosen carefully for each basic composition and annealing temperature, Figure 11.
| Figure 11 - Solubility of cobalt in 14 and 18 carat gold alloys |
Effects
Besides grain refining, Figures 12 and 13, cobalt increases the hardness of age hardened alloys to some extent.
Side effects
If the solubility of cobalt at solidification is exceeded, coarse segregations can be formed. These nests of particles detrimentally affect the polishing behaviour. In addition to the cobalt content, the speed of solidification plays a role: Slow solidification increases the size of the particles, Figure 14.
Recommendations for application
Cobalt additions can be used successfully for grain refining during soft annealing, provided the concentration is adjusted to suit the basic composition of the alloy. Grain refining during casting, using increased cobalt additions, can cause detrimental segregations of cobalt particles which affect alloy properties.
Conclusion
Relatively few elements have proved useful in practice as additions for improving the properties of 14 and 18 ct yellow gold alloys. Zinc is the addition which will give the least problems if the concentration is not too high and some simple precautions are observed.
Silicon is also very effective for improving casting properties. However there are serious side effects. These effects can cause many problems and they are difficult to avoid. Precise control of the small addition to match the alloy composition is essential if embrittlement is to be avoided.
Iridium has been established as a grain refiner during casting. The maximum concentration is small and has to be carefully controlled and some care has to be taken in the preparation of the alloy. Cobalt is an effective grain refiner during soft annealing. Other grain refiners are mentioned in literature but are seldom used in practice.
Other small additions such as titanium, rare earth metals, calcium and barium are used in high carat gold and in micro alloyed 'pure' gold for improved strength and hardness.
References
- D. Ott and C.J. Raub, Metall, 34, 1980, 629-633
- D. Ott and C.J. Raub, Metall, 35, 1981, 543-548
- D. Ott and C.J. Raub, Metall, 35, 1981, 1005-1010
- D. Ott and C.J. Raub, Metall, 36, 1982, 150-157
- L. Gal-Or and M. Riabkina-Fishman, Proc. Santa Fe Symposium, 1987, Met-Chem Research Inc, 125-141
- D. Ott and C.J. Raub, Gold Bulletin, 14, 1981, 69-74
- D. Ott, Gold Technology, No 22, 1997, 31-38
- C.J. Raub and D. Ott, Gold Bulletin, 16, 1983, 46-51
- D. Ott and C.J. Raub, Gold Technology, No 7, 1992, 2-7
- G. Normandeau, Proc. Santa Fe Symposium, 1996, 83-87
- J.C. McCloskey, P.R. Welch and A. Shankar, Gold Technology, No 30, 2000, 4-7
- J.C. McCloskey, P.R. Welch and A. Shankar, Gold Bulletin, 34, 2001, 3-13
- G. Normandeau and R. Roeterink, Gold Technology, No 15, 1995, 4-15
- D. Ott and C.J. Raub, Gold Technology, No 7, 1992, 2-7 15 D. Ott, Gold Technology, No 22, 1997, 31-38
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.