Minerals and Crystals Systems
A Mineral may be defined as a homogenous substance produced by the processes of inorganic nature having a chemical composition and physical properties which are constant within narrow limits. Its structure is crystalline. It is composed of the same substance throughout. Except for impurities it has the same chemical formula for all specimens of the mineral. Its atoms usually have a definite and ordered crystal structure. What makes a mineral.
5 Minute Read
This article is an introductory listing of definitions and nomenclature concerning gem materials.
A Mineral may be defined as a homogenous substance produced by the processes of inorganic nature having a chemical composition and physical properties which are constant within narrow limits. Its structure is crystalline.It is composed of the same substance throughout. Except for impurities it has the same chemical formula for all specimens of the mineral. Its atoms usually have a definite and ordered crystal structure.
What makes a mineral (or an organic product) a gemstone is cultural and partly subjective: beauty, durability and rarity.
Minerals often occur in geometrical forms bounded by plane surfaces. These are crystals and the internal structure determines properties which allow the identification of the gem material; its differentiation from other minerals, imitations and sometimes synthetics.
Crystals have:
- An orderly and symmetrical atomic structure.
2. A definite external geometrical shape bounded by plane faces.
3. Physical (and optical) properties which vary with direction.
Glass has:
- No regular atomic structure.
2. No tendency to assume a definite external shape.
3. Properties which are the same in all directions.
(the above derived from the British Gemmological Association's course material. I strongly recommend their program to aspiring gemmologists)
Crystalline Material: Possesses the regular structure and directional properties of a crystal but not the regular geometrical shape. Also called massive. e.g. rose quartz.
Crypto-crystalline Material: Material which consists of a multitude of tiny, often submicroscopic crystals. e.g. Chalcedony.
Symmetry
Crystal symmetry refers to the balanced pattern of the atomic structure which is reflected in the external (crystal) shape. Different species vary in the symmetrical arrangement of faces. These arrangements have certain 'planes' and 'axes' of symmetry. These form part of the definition of the crystal system to which specific gemstones belong.
Plane of Symmetry
An imaginary plane dividing a body into two parts such that each is the reflected image of the other. Crystals may have more than one plane of symmetry. i.e. a cube has nine planes of symmetry.
Axis of Symmetry
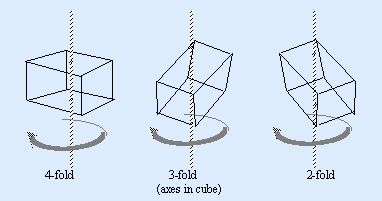
An imaginary axis is placed through a perfect crystal so that during a single rotation about this axis the outline of the crystal form appears identically more than once; 2, 3, 4 or 6 times.
Centre of Symmetry: (Centro-Symmetry)
Often present, it exists when every face of a perfect crystal is exactly opposite a similar face on the other side of the crystal.
Crystal Axes: (Crystallographic Axes)
To describe crystals imaginary lines are used intersecting at 0 (the origin). These are specific to the various crystal systems, intersecting at given angles and being of given lengths specific to each crystal system.
Origin
The intersection of the crystal axes.
Habit
Gemstone species tend to occur in characteristic shapes which relate to one or more of the forms common to the crystal system of the gemstone in question. The crystal form or forms which a gemstone most often appear are it's habit. e.g. diamond: octahedron, emerald: 6 sided prism.
Form
Those faces of a crystal which are identically related to the crystal axes. When the space so defined is completely enclosed (cube, octahedron) it is a closed form. When identical faces do not completely enclose the space (four or six sided prism; top and bottom open) it is an open form.
Twinned Crystals: (Compound Crystals)
A twin is a single crystal composed of two or more parts with any part in reversed structural orientation to the next, or interpenetrated.
Contact Twin
Sharing a common plane.
Interpenetrant Twin
Two individuals have grown from a common origin and appear to penetrate each other. e.g. cross stones.
Lamellar Twinning
A series of contact twins often as extremely thin plates. Atoms in adjacent sheets are reversed, i.e. alternate plates are in the same order. This can give rise to special optical effects as in the feldspar labradorite.
Secondary Twinning or Parting
The crystal is composed of very thin plates parallel to definite crystallographic directions. e.g. ruby, this gives rise to 'false cleavage'.
CRYSTAL SYSTEMS
Cubic
Three crystal axes of equal length intersect at right angles to each other. e.g. diamond, spinel, garnets. Tetragonal Three axes intersect at right angles to each other. The vertical axis is of unequal length while the two horizontal axes are of equal length. e.g. zircon, rutile.
Hexagonal
Four crystal axes. Three are of equal length and intersect at 60o to form a horizontal plane which the fourth intersects at right angles. The vertical fourth is of unequal length and forms an axis of 6-fold symmetry. e.g. Beryl, apatite.
Trigonal
Four crystal axes. Three of equal length intersecting to form a horizontal plane which is intersected at right angles by the fourth axis. The vertical fourth is of unequal length and forms an axis of 3-fold symmetry. e.g. quartz, corundum, tourmaline, dioptase, haematite.
Orthorhombic (Rhombic)
Three crystal axes of unequal length interest each other at right angles. e.g. topaz, peridot, Chysoberyl, iolite, sinhalite, andalusite.
Monoclinic
Three axes. Two of unequal length intersect each other obliquely to form a plane which is intersected by the vertical third (of unequal length) at right angles. e.g. jadeite, nephrite, diopside, orthoclase feldspar, serpentine, sphene, malachite, spodumene.
Triclinic
Three axes of unequal length intersect each other at oblique angles. e.g. turquoise, labradorite.
NOTE: The Gemological Institute of America (GIA) system for crystal types differs from the above. Contact their web site for information on their courses, books and so on.
CRYSTAL SYSTEM SYMMETRY
Singly Refractive: Amorphous {no crystal structure)
| Optic Axis | |||
| Cubic | 9 planes | 4 3-fold | |
| 13 axes | 3 4-fold | ||
| a centre | 6 2-fold |
Doubly Refractive
| Optic Axis | |||
| Tetragonal | 5 planes | 1 4-fold | uniaxial |
| 5 axes | 4 2-fold | ||
| a centre |
| Optic Axis | |||
| Hexagonal | 7 planes | 6 2-fold | uniaxial |
| 7 axes | 1 6-fold | ||
| a centre |
| Optic Axis | |||
| Trigonal | 3 planes | 3 2-fold | uniaxial |
| 4 axes | 1 3-fold | ||
| a centre |
| Optic Axis | |||
| Orthorhombic | 3 planes | 3 2-fold | biaxial |
| a centre |
| Optic Axis | |||
| Monoclinic | 1 axis | biaxial | |
| a centre |
| Optic Axis | |||
| Triclinic | no planes | biaxial | |
| no axes | |||
| a centre |
Uniaxial
The optic axis of the crystal is parallel to the main crystal axis. One direction of single refraction.
Biaxial
There are two directions of single refraction. (optic axes)
GEMSTONES BY CRYSTAL SYSTEM (major ones)
*Diamond simulants, man-made (U) = uniaxial, (B) = biaxial
Cubic
| Orthorhombic (B)
|
Tetragonal (U)
| Trigonal (U)
|
Monoclinic (B)
| |
Triclinic (B)
|
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
Charles Lewton-Brain
Master goldsmith Charles Lewton-Brain trained, studied and worked in Germany, Canada and the United States to learn the skills he uses. Charles Lewton-Brain is one of the original creators of Ganoksin.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.