Electroforming Step-by-Step
Electroforming is the process of controlling the metal deposit from an anode metal, through electrolyte solution, onto an electroconductive surface. Basically, a skin of metal is built up into a rigid structure. The materials you will need to get started are as follows.
4 Minute Read
Electroforming is the process of controlling the metal deposit from an anode metal, through electrolyte solution, onto an electroconductive surface. Basically, a skin of metal is built up into a rigid structure.
The materials you will need to get started are as follows:
- 10 Amp Rectifier
- Clamp and lead set 8 gauge wire for bus bars
- 18 gauge copper wire
- Electroconductive paint
- Bath containers (Pyrex plastic, acid resistant, with lids, from a restaurant supply company; these have measurements marked on the side)
- Copper electroforming solution
- Electroclean solution [*]
- Replenishing brightener [*](allows electrolyte solution to maintain a bright finish)
- Acid dip solution [*] (non-cyanide)
- Stainless steel anode
- Red lacquer - block out resist
[*]Refers to the items needed for electroforming on copper; can be purchased from Rio Grande or plating and jewelry supply stores.
Step 1
Select a workpiece. Any surface that is conductive and can stand up to the acids in electrolyte solution will work. Organic and porous materials, such as a leaf or paper, need to be sealed with several coats of varnish. Wax molds and plastic work well, also. Red lacquer can be used a block out resist. Paint it on the areas you don't want electroplated / electroformed. Use on organic materials and gemstones. At this point, you will attach the 18 gauge wire to your workpiece. Hot glue or epoxy work well, and allow for a good connection during the electroforming process. If there are multiple protruding areas, you may not want to glue; rather, wrap the wire around your workpiece, making sure there is contact. Later, you can adjust the points of contact, repaint the spots, allow to dry, and then submerge back into the solution.
Step 2
Clean your piece in an alcohol solvent. Be sure to avoid skin contact with the piece, as the oils in skin may hinder electrodeposit. You are now ready to apply the electroconductive paint. This can be purchased from electronic stores or online. Spray or brush an even coat of paint.
Note: Brush strokes will plate as such. Use lacquer thinner if your paint thickens. Allow two hours to dry.
Step 3
Prepare your anode. Use a clean piece of copper (scrap sheet). It should be equal in surface area to your workpiece. Suspend it in the electrolyte solution one of two ways: 1) Drill hole in copper and run your 18 gauge copper wire through, twist tightly, and wrap around bus bars, then attach alligator clip. This works well when doing multiples; or; 2) Bend copper over side of container and attach clip. This works well for a large piece in a large tank. You should occasionally move and adjust your workpiece within the solution.
Step 4
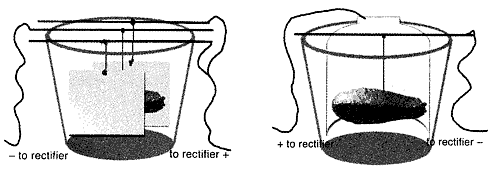
Suspend your piece from the bus bar (in the center - Figure A). It is important to make sure the anode and the cathode (workpiece) Do Not Touch . Attach your clips, negative to your workpiece and positive to the anode. If your clips are rusting or have a build-up of salts from the solution, use a wire brush or file to clean them off, avoiding contamination and maintaining a strong connection.
Step 5
Turn your rectifier on. A slow build-up of metal will give you the best results and details. Use low voltage to achieve this (1 volt or less). The higher the current, the grainier the deposit will be. This can make for interesting texture, yet can cause crumbling if done too quickly.
Check your piece periodically and make the necessary adjustments until you have obtained the desired weight. Protruding areas and angles will build up deposits faster than curved areas. A small piece can take from 2 - 12 hours or more.
Experimenting with your setup will teach you how to control your results.
Step 6
Turn off the rectifier. Remove your piece from the solution and rinse under water. Dunk in Acid dip and stir for a few seconds to clean, then rinse in water again. Neutralize in baking soda/water solution. Your piece will have a pink cast and will oxidize quickly. Electroformed objects can be soldered, patinaed and enameled. It will not withstand much forming. Use a torch or kiln to burn out your object. If you are using wax, it can be boiled in water for about 20 minutes to remove it. Hollow formed objects should have a hole drilled into it to vent during burnout. Remember to take the correct safety precautions using the solution. Dispose of the hazardous materials properly and have plenty of ventilation in your workspace.
Electroforming on Copper
Prepare your piece by cleaning it and attaching your lead wire to negative, step 1 above. Clip positive to your stainless steel anode and immerse into the electrocleaning solution. Turn on your rectifier and let agitate for 1 - 2 minutes. The higher the volts, the more bubbles you will see. Remove clip, and do a water rinse. Dunk in Acid dip for 15 - 30 seconds, then rinse in water. Submerge your workpiece into the electrolyte and attach to the negative lead. Attach positive to the anode. Turn the rectifier on low.
The results you can achieve from the electroforming process can be quite exciting. Use this system for wax molds, one-of-a-kind pieces, or just to add some texture. Work with your set-up to explore the possibilities, and have fun!
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.