Inquartation and Parting Refining Process
The small-medium scale refiner (typically up to 3-4 kg per batch) tends to adopt the Aqua Regia process as being a relatively straightforward technique, capable of producing gold with a purity in excess of 99.9%. The essential steps in the process are to dissolve the refinable material (such as jewellery process scrap) in aqua regia, a mixture of strong nitric and hydrochloric acids, and to selectively precipitate the gold from the resulting solution.
8 Minute Read
The small-medium scale refiner (typically up to 3-4 kg per batch) tends to adopt the Aqua Regia process as being a relatively straightforward technique, capable of producing gold with a purity in excess of 99.9%.
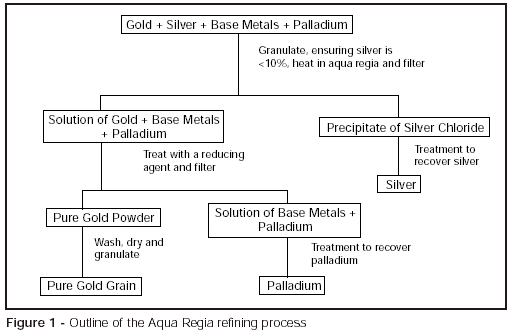
The essential steps in the process are to dissolve the refinable material (such as jewellery process scrap) in aqua regia, a mixture of strong nitric and hydrochloric acids, and to selectively precipitate the gold from the resulting solution. Gold and most other metals will dissolve in aqua regia; silver, however, will form a silver chloride precipitate. Because of this reaction of silver, it is necessary to restrict the amount of silver in the start material to be refined to a maximum of 10%; any more than this will run the risk of the silver chloride preventing complete dissolution of the gold by masking it off. It is necessary, therefore, to blend batches of high silver content material with those of low or no silver content prior to dissolution or to treat with nitric acid initially to remove silver if, for example, individual batches cannot be blended together.
To aid dissolution in aqua regia, it is usual to melt and granulate the material first (essential when lots are blended), to provide as large a surface area as possible, and to heat the acid to speed up the dissolution process. Silver chloride is removed by filtration. The solution is then treated with a reducing agent to precipitate pure gold. Of the reducing agents available, ferrous sulphate, sulphur dioxide and sodium bisulphite are most widely used. In this way, high purity gold powder is produced which needs to be filtered, washed and dried and then is usually melted and granulated, ready for subsequent use.
If any palladium is present in the original material, as might be the case for white gold scrap, palladium will dissolve in aqua regia but will remain in solution when the gold is precipitated and can be recovered separately.
The refining process is illustrated schematically in Figure 1.
The technique does have some disadvantages in that small, but significant, losses of gold are likely to occur in the dissolution and filtering stages and in the handling of the powder product. Furthermore, the equipment, of necessity, is made in glass so as to withstand the aqua regia. There are also environmental considerations which need to be addressed because of the emission of gases (nitrogen oxide, hydrogen chloride and sulphur dioxide) and solutions are produced which can contain nitrates, nitrites, chlorides, sulphides and sulphates and which have to be disposed of. Nevertheless, for the manufacturers/refiners who are dealing largely with high carat gold alloys (18 carat and above), then this process is probably the most economic and several equipments are available commercially which can treat several kg per day.
However, for lower caratage alloys, inquartation and parting becomes a most attractive alternative process and this paper sets out the principles of the technique and then outlines an equipment that has recently become available, together with results of a refining trial using 9 carat gold scrap.
Inquartation and Parting
The technique mirrors part of the Fire Assay process used in the analysis (assay) of gold.
Inquartation involves initially diluting the gold in the refinable material to about 25% (literally 'quartering' the gold) by melting with the appropriate addition of copper or silver, granulating the melt so as to generate a high surface area and then treating with nitric acid. The acid will dissolve the silver and base metals, leaving behind the gold. The acid treatment is known as parting as it 'parts' (separates) the gold from the silver and base metals. Dilution of the gold content is necessary to ensure that the nitric acid can readily attack and dissolve the base metals and silver, some of which may be otherwise masked by the gold.
Any palladium which is alloyed in the gold will also be removed by the acid. The process is depicted schematically in Figure 2.
Advantages that the technique has over the aqua regia process are that it is a much simpler operation in which no loss of gold should occur as base metals, silver and palladium are dissolved out of the refinable grain, leaving all the gold behind, still in its original grain form. The equipment can be made in stainless steel which is obviously more durable than glass. Nitrogen oxide is evolved which has to be dealt with whilst the solution generated at the end of the process contains nitrates which can be treated to recover silver and palladium, leaving base metal nitrates for disposal.
Pirotechnia Aure8 Refining Equipment
The Aure8 equipment, manufactured by Pirotechnia srl, Italy, is modern and compact with a capacity of about 3-4 kg of grain (gold content 25%) and which operates in a closed circuit without any atmospheric emissions over a 6 hour cycle. The equipment is shown in Figure 3 and has dimensions 2.05 x 1.1 x.0.76 m. The major components are:
- An 8 litre capacity stainless steel reactor, which is heated by an immersion heater; a cooling system for the reactor; a viewing port in the reactor through which the charge is introduced.
- A condenser for condensing some of the gaseous products.
- A gasometer for collecting the nitrogen oxide reaction product and converting it back into re-usable nitric acid.
- A storage tank for collecting the liquid reaction products.
- A filtering system for separating the refined gold from the liquid reaction product.
- A storage tank for nitric acid.
- An electromagnetic pump for enabling liquids to be pumped around the circuit.
- A separate control panel provided with switches for a digital pyrometer, heater and pump.
- Stainless steel pipework.
Material requirements are nitric acid, water for cooling, de-ionised water, compressed air and oxygen from a gas cylinder. The ideal starting material for refining is grain of 2-3 mm diameter and a gold content of 25% or less. Thus, carat gold scrap jewellery or production scrap needs to be diluted by melting with the necessary amount of copper (or silver) and then granulated. Larger grain particles will require longer refining times than the recommended 6 hour cycle in order to achieve purities of 99.9% and higher. Higher starting gold contents run the risk of incomplete refining although, depending on the operator's requirements, lower purities may be acceptable.
The grain is charged into the reactor ready for parting. As in the fire assay procedure, parting is a 2 stage operation. Initially, dilute nitric acid is fed into the reactor over a 30 min. period to ensure the reaction is not so violent as to destroy the grain and form a fine particulate material. The solution is then boiled for 2 hours, cooled and drained. The operation is repeated with a slight excess of strong nitric acid before finally the grains are discharged and washed.
A third parting operation may be applied which could lead to an improved gold purity of 99.99%.
Nitrogen oxide gas is collected in the gasometer and converted back into nitric acid by dissolving in de-ionised water in the presence of oxygen. This acid is concentrated over several operations before being re-used. Hence, there are no gaseous emissions from the plant.
The drained solution will comprise largely silver and copper nitrates plus other base metal nitrates. Any palladium in the start material will also be present in the solution. Recovery of silver can be effected by adding caustic soda to the solution to neutralise the excess nitric acid, then adding pieces of metallic copper which will react with the silver nitrate to precipitate silver. Any palladium present will also be precipitated with the silver and so, in that case, subsequent separation of silver and palladium would be necessary, probably by an external refiner.
Refining Trial
A trial was carried out in the Aure8 equipment at the Birmingham Assay Office in late 1998. The starting material was mixed 9 carat yellow gold scrap which was initially melted with sufficient copper to dilute the gold to about 25% and then granulated. The granulation was not specifically controlled to give the optimum grain size (2-3mm diameter) and resulted in a range of grain sizes up to 6 mm. Samples of the grain were assayed and then the refining procedure carried out in 2 stages, as described earlier.
The refined grain was washed, dried and weighed and a number of samples taken for assaying. All assays were carried out by the Birmingham Assay Office. Details are given in Table 1. The trial was completely successful in that, despite starting with less than ideally sized grain, satisfactory refining was achieved with essentially no loss of gold.
After sampling and assaying, the residual gold grain was melted in a crucible and cast into an ingot. A bar weighing 290 g was obtained which assayed at 99.97% purity, which kg per day) of largely 14 ct or lower materials, then it is likely that this technique will prove more economic than the aqua regia process. Where the material is largely 18 carat or higher, the process becomes somewhat less attractive because of the additional quantitites of copper or silver which need to be added to achieve a starting gold content of 25% for refining, although the process will still operate perfectly satisfactorily. suggests that by optimising the initial grain size, it could well be feasible to produce 99.99% gold in the equipment. The apparent increase in gold assay figure on melting the refined grain can probably be attributed to the grain assay being the average of values of several individual grains and to the possibility of incomplete washing of the grain.
Discussion and Conclusions The inquartation and parting gold refining technique is capable of producing up to 99.99% pure gold and is considerably easier to operate than the aqua regia technique on a small scale. It is eminently suited to the refining of low carat gold alloys, including white golds, and for those manufacturers and refiners dealing with relatively small quantities (a few kg per day) of largely 14 ct or lower materials, then it is likely that this technique will prove more economic than the aqua regia process. Where the material is largely 18 carat or higher, the process becomes somewhat less attractive because of the additional quantitites of copper or silver which need to be added to achieve a starting gold content of 25% for refining, although the process will still operate perfectly satisfactorily.
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.