Casting Investment Powder Performances
In each step of manufacturing jewelry, there are many factors that can adversely affect quality. Because of this, process control is very important. Investing is one of those steps in which process control is vital. Most investment manufacturers help the investor control the process variables by providing a consistent product, user instructions and technical support. However, improper handling of the investment powder often destroys their efforts. Improper storage, poor water quality, wrong mixing ratios, insufficient mixing methods, uncontrolled temperatures and others can each reduce casting quality and thus cause loss of production and money. This paper will present some data that will show what some factors do to the various investment properties.
21 Minute Read
This article "Casting Investment Powder Performances" focuses on the process, measurement and mixing ratio and time for casting investments.
Introduction
In each step of manufacturing jewelry, there are many factors that can adversely affect quality. Because of this, process control is very important. Investing is one of those steps in which process control is vital. Most investment manufacturers help the investor control the process variables by providing a consistent product, user instructions and technical support.
However, improper handling of the investment powder often destroys their efforts. Improper storage, poor water quality, wrong mixing ratios, insufficient mixing methods, uncontrolled temperatures and others can each reduce casting quality and thus cause loss of production and money. This paper will present some data that will show what some factors do to the various investment properties [1, 2] and discuss why these are important. The specific factors that will be focused on are: water quality, the temperature of the water and of the powder and also the water-to-powder mixing ratio. This paper will show how dependent are the properties, and thus the performance of gypsum-based investment, on these factors. To help in understanding the data, some background information on gypsum investments and the test methods employed needs to be covered.
Background
Gypsum-based jewelry investments contain three classes of materials, refractory material, bonding material and controlling chemicals. Refractory materials can withstand high temperatures without decomposing. Silica serves this function. Bonding materials are what hold the refractory materials in place to form a mold. This is the purpose of gypsum. Controlling chemicals are used to control how quickly the bonding materials set up and to accentuate various investment properties.
The bonding material, gypsum, is actually formed by the chemical reaction between water and plaster [3]. Water molecules chemically bond with plaster molecules to form gypsum. As with most chemical reactions, the rate in which it proceeds is highly dependent on temperature, interfering compounds, the condition of the reactants and the amounts of the reactants [4]. Investment manufacturers make great efforts to assure that this reaction proceeds at the designed rate. They achieve this by holding the raw material suppliers to strict standards of purity, quality and consistency. They also achieve this by using the controlling chemicals. However, compounds that affect the rate of reaction are often found naturally in water [5]. These interfering compounds can negate the effort manufacturers put towards making a consistent product.
Testing Procedures
Pour Time
Pour time is similar to working time, but there is a subtle difference. The working time is the amount of time from when all the powder is added to the water until the investor feels that the mixture is too viscous to continue to work or manipulate [6]. It is easy to understand how this will depend on the user's preference and the application. On the other hand, the pour time can be described in a less subjective manner. The pour time is the amount of time it takes the investment to become so thick it will not pour. It is a measurement of reaction time. For repeatability, the investment is mixed with a specific intensity and specific time and the pouring is done precisely every 15sec [7].
Set Time
The set time is the amount of time it takes the investment to become so hard that the Vicat needle will not penetrate more than 1mm. The Vicat needle is 1mm in diameter and has 300gm weight behind it [7]. At this point, the investment is about 22% of its full strength [6]. It will take 1-2hrs longer before it is hard enough to disturb by further processing, such as transferring to a burnout oven.
Slump
To measure the fluidity of an investment, a slump test is used. To measure the slump of an investment, it is mixed in the same way as for pour time and set time. Some material is poured into a cylinder, 5cm tall and 3.5cm diameter, standing on a glass plate. The top is struck off level. At exactly the 2min mark, the cylinder is lifted off the glass [7]. The investment material will drop out and spread out equally in all directions. The thinner investments will result in a larger disk (spread diameter) than the thicker ones.
Strength
The green compressive strength refers to the amount of pressure needed to destructively compress the investment 2hrs after it had set. Fired compressive strength refers to the amount of pressure needed to destructively compress the investment after it has been through burnout and then cooled slowly to room temperature. The firing process weakens gypsum investments tremendously. Some investments are weakened more than others, depending on controlling chemicals, particle sizes, raw material percentages and plaster grades.
In order to obtain accurate and repeatable compressive strength results, it is critical to closely adhere to a consistent set of procedures [7]. The results can be affected by length of mixing time, mixing intensity, whether a vacuum step is included, air bubbles, unparallel ends and disturbing the molds too soon.
Castings
Castings were made at several water-to-powder ratios. The investment was mixed until the 3min had elapsed and then it was vacuumed for 20sec. The 10cm diameter by 12.7cm high flasks were filled to the top of the pattern and vacuumed for 1min. The flasks were then filled the rest of the way and set aside for hardening. The recommended burnout schedule was used [8]. The flasks were at 510°C when cast. A brass alloy of 60% copper and 40% zinc was centrifugally cast at 1091°C. The same pattern was used for each water-to-powder ratio.
Results & Discussion
Water quality
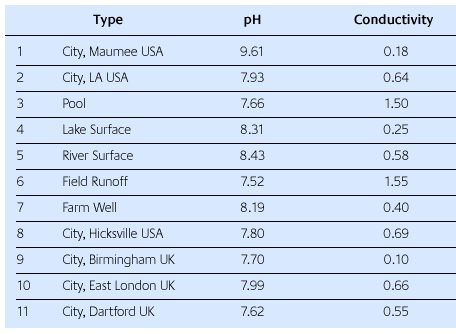
In order to see how much the investment properties are dependent on water quality, samples of water from various sources were obtained. The point of examining all of the water sources is not to predict what any particular water source might be like. Each water source is different and will change with time. The point is to simply use several different water sources from anywhere.
In Table 1, it can be seen that the acidity (as measured by pH) of the waters varies considerably as does as the conductivity. Conductivity is simply the inverse of electrical resistance. Pure water does not conduct electricity. Its resistance approaches infinity. The inverse of infinity is zero, so the conductivity of really pure water is zero. But when compounds dissolve in water, their ions give water the ability to conduct electricity [9]. Here the unit of conductivity is millimhos.
The main observation from this table is that each water source is different in terms of pH and conductivity. Using a single investment powder, each water source was used to make an investment mixture and test its pour time, set time and slump. The results obtained were then compared to a standard investment mixture made with de-ionized water from R&R's research facility.
De-ionizing is simply a method of water purification. It does what its name suggests, that is, it removes ions from the water. The investment properties obtained with the standard mixture were subtracted from the investment properties obtained with each water source. This was done because the difference from the standard is what is of interest. That is how the data is shown in the following graphs.
Figure 1 show what happened to the pour time and set time of each investment. On the y-axis is the difference between the standard and the result caused by a particular water source. On the x-axis is the water source reference number. The standard is represented by zero or the x-axis. Every water source caused the pour time and set time of the investment mixture to be longer than the standard. If a source was shorter than the standard, the bar would dip into the negative region. Some varied from the standard more than others.
What this means to the investor is that the working time and setting time are longer. In one instance we had a customer once who had a setting time greater than 24hrs. They never had problems like this before, but their water quality changed on them without their knowing. This brings up the point that water sources can change with time. So a water source may not cause trouble now but it may tomorrow or next week. The point of this is that if the water is changing, then the pour time and set time are changing and the investor is left guessing as to when it is appropriate to load the flasks into the oven.
The measurement of fluidity is the slump test. Figure 2 shows the degree of slump versus water source. Here again on the y-axis is plotted the difference from the standard. The standard is the x-axis. Notice that some water sources did not affect the slump at all. Source number seven made the slump larger than the standard. Most of the others made the slump smaller. Each water source affected the slump in its own way. Now do not get concerned about which water source most represents your water. The point here is that each water source does something different. As a result, this may cause casting defects, such as trapped air, watermarks or rough surfaces.
The water quality can change the designed pour time, set time and fluidity of the investment. This relationship is a source of variability in mold making. Because of this variability, the amount of working time is no longer known. The time when the molds are ready to be loaded into the oven is no longer known. Defects may occur due to the effect on the fluidity.
So what can be done to help this situation? The investment manufacturers suggest that de-ionized water be used, when mixing their product [8]. In order to test whether their suggestion works, each water sample was de-ionized, another investment mixture made with it and the tests run again.
Figure 3 is a graph of the pH of the water samples before de-ionizing (in red) and then after (in green). The pH in each case came down.
Before the water samples were de-ionized, each sample could conduct electricity quite welled-ionizing reduced the conductivity to zero, as shown in Table 2. The de-ionizing process removed ions. This is all nice but what does it do to the investment properties?
Figure 4 is a graph of the properties of the same investment as measured with the same water sources. Only now each water sample was de-ionized. Remember the y-axis is the difference from the standard. That means that the standard is at the zero line. The same standard was used as before. The range on the y-axis was kept the same as before to give perspective. Each water source gave essentially the same pour time and set time.
Close inspection will show a very small deviation from the standard for mixtures made from waters numbered 4, 5 and 6. Included are error bars to represent the accuracy of the test. These error bars relate an idea of how small the variation really is. The small variation of number 4, 5 and 6 are absorbed by the variation inherent in the test. Therefore, the variation of 4, 5 and 6 are really quite negligible.
Remember that before, each water source caused the pour time and set time to deviate from the designed. By simply de-ionizing, all variation associated with each water source was removed. This is true with every water source tried. This suggests that, regardless of the beginning water condition, by de-ionizing, the investment properties will have the designed values.
Figure 5 shows the slump as a function of de-ionized water sources. The slump can be measured to ±0.16cm.The error bars reflect that. When considering the accuracy of the test, what little variation that remains is really negligible.
Reviewing the observations, de-ionizing reduced the pH and conductivity. The conductivity measurement is a good indication of whether the water is good enough. The lower the better. The variability in pour time, set time and slump was reduced. This means that the investing process would be more consistent and reproducible. That means more consistent castings. That means fewer worries, less reworked material, less scrap and less cost. The customer, mentioned earlier, who had a greater than 24hr set time, was recommended to de-ionize his water and it was observed that the set time went back to normal.
Temperature
The next factor explored was temperature. How does the temperature of both the water and investment powder affect investment properties? In order to determine this, the investment properties were measured several times, varying only the starting temperature, which is the temperature of both the water and powder prior to mixing together. De-ionized water and the standardized test procedures were used each time.
Figure 6 shows the results. On the x-axis is the starting temperature. The y-axis has actual time and not a difference as in the previous graphs. The red line represents the pour time as a function of temperature and the green line is the set time.
In the temperature range shown on the graph, the pour time changes by two minutes. The set time changes by a little over three minutes. What this means is that if the temperature is not controlled, then there would be an uncontrolled swing in the pour time and set time.
This dependence on temperature may allow some investors to change the amount of time used to invest. An extra minute may be needed in certain applications or possibly the investor would like to slightly speed up the setting. The investor can do this by adjusting the temperature.
Figure 7 shows the fluidity versus temperature. The slump results were fairly constant even though the starting temperature was changed. The slight differences are negligible, when considering the precision of the test. This is reassuring to know. If the temperature is changed to over the setting time a little, then there need be no concern about causing problems associated with a change in fluidity.
In review, the pour time and set time are reduced as the temperature is increased. The fluidity is not very dependent on temperature. If the temperature is controlled, then the pour time and set time are controlled to some degree. If the temperature is not controlled, then the investment's pour time and set time will vary.
Water-to-Powder Ratio
The last factor explored is the water-to-powder ratio. How does the amount of water used to mix investment affect the investment properties and ultimately the castings? Before answering this, it is important to know exactly what water-to-powder ratio is.
The best way to explain this might be to give an example. Commonly used in the jewelry industry is a water-to-powder ratio of 40ml of water for every 100gm investment powder. It is simply a ratio of how much of ingredient A to mix with ingredient Bathes gets confused because it can be written in several ways, like 40:100, 40/100, 40, 0.40 and 28.57%water.Each of these is trying to convey the same water-to-powder ratio.
Back to how the water-to-powder ratio affects investment properties. To answer this, several investment properties were measured at several water-to-powder ratios. Castings were also made at several water-to-powder ratios in an attempt to observe detrimental effects. A premium investment powder was used for the tests. This is an important detail, as will be shown shortly. All measurements were made at 22.2°C - 23.3°C and with de-ionized water. All variables were held constant, except the water-to- powder ratio, which was varied from 34 to 46 (i.e. 34:100 to 46:100). The recommended water-to-powder ratio for this product is in the range 39-42, so some of the tests were outside the recommended water-to-powder ratio range [8]. The testing procedures used were from R&R's time honored ISO 9002 quality procedures [7].
Following are a series of graphs that are very similar. Each graph will show at the top what property it relates to. Each graph will have the water-to-powder ratio at the bottom, along the x-axis. The range of the x-axis is the same on each graph, which also has a box showing where the recommended water-to-powder ratio range is. Along the y-axis is the measured property and its units.
In Figure 8, it can be observed that the pour time is directly proportional to the water-to-powder ratio. There is actually quite a lot of change realized in the pour time. In the recommended range, 39-42, the pour time changes by 1.25min. This provides a moderate amount of flexibility for the investor who needs just a little extra time to complete all the process steps. In applications where this extra 1.25min is not enough, then possibly other process parameters should be looked into, rather than venturing out of the recommended water-to-powder ratio range. These other process parameters include mixing time, vacuum time and the number of flasks invested at any one time.
Figure 9 shows the set time as a function of water-to-powder ratio. In the recommended water-to-powder ratio range, the set time changes by about 1.5min. It is desirable to have a rapid setting after the flasks are filled. Otherwise, the chances of watermarks are increased [10]. For most investments, this is a serious problem; however, some will not cause watermarks regardless of set time.
Figure 10 shows the fluidity as a function of water-to-powder ratio. As expected, the investment gets thinner with increased water-to-powder ratios. What is more interesting is the magnitude of the change in the recommended water-to-powder ratio range. It could be said that the slump is more dependent on water-to- powder ratio than pour time and set time. The pour time about doubled from the lowest water-to-powder ratio to the highest water-to-powder ratio. The set time did not quite double. Here the slump diameter doubled, making the area of the disk increase by close to five times.
The fluidity might be the one property most people want to change to their liking. It is thought that a more fluid mixture is needed to get better detail reproduction and better fill [10, 11]. Figure 10 definitely shows that by increasing the water-to- powder ratio you can achieve a more fluid mix. Within the recommended water-to-powder ratio range, plenty of fluidity change can be experienced to meet most needs. From a change in water-to-powder ratio from 39 to 42 the area increases by 1.37 times. By going outside the recommended range, changes in the other properties to a significant degree will create more headaches.
Figure 11 shows the green compressive strength as a function of the water-to-powder ratio. This relationship is inversely proportional. There is a slight flattening of the curve in the middle. This indicates a less dramatic change in the green compressive strength near the recommended range and larger changes outside the recommended range. What this means is that there is some ability to change the green compressive strength, while within the recommended water-to-powder ratio range. Outside this water-to-powder range, the change in green compressive strength is more severe, the slope is greater, and may cause defects.
The weakening due to a high water-to-powder ratio may contribute to cracks or a rough surface finish [10,11]. I have actually seen in one application how a sandy surface occurred on close to 80%of the castings and when the water-to-powder ratio was reduced by only 1ml/100gm the defect went away. When a process is on the edge of the recommended range, it may only take a slight change in the water-to-powder ratio to cause problems. This idea is shown on the graph. It is like rolling or falling off a cliff.
Figure 12 shows the fired compressive strength as a function of water-to-powder ratio. Because this is measured after firing and cooling to room temperature, it is more of an indication of the strength after casting. Therefore, it may impart some information on the ease of removing the casting from the mold. This is, of course, not the only factor affecting removal. There are formulation factors as well.
The dramatic increase at the lowest water-to-powder ratio could cause difficult removal of the casting. As with other properties, there is realized some flexibility within the recommended water-to-powder ratio range. The investment is designed for this range. By going outside the range, problems may arise.
The following are a few observations obtained while investing and casting at the various water-to-powder ratios tested. At higher water-to-powder ratios it was noticed that the investment would splash out of the mixer at normal speeds. This created a mess and called into question whether a good mix was achieved. After mixing, during the vacuum steps, the investment rose much higher than usual. This could potentially cause more housekeeping problems and loss of investment, unless a very tall collar is used. The chemicals added to control this were probably diluted too much by the excess water to be effective. However, the castings did not have watermarks or fins. All around they looked good.
At the lower water-to-powder ratios, the investment was really difficult to pour. The metal filled all the way, there were no air bubbles trapped, the mold may have broken away less than at high water-to-powder ratios, but it was still very good and all around the castings looked fine.
Why were there no defects? Conventional wisdom suggests that problems should have arisen. At high water-to-powder ratios there should have been cracking that gives way to fins, rough (sandy)surfaces and watermarks [10,11]. At the lower water-to- powder ratios there should have been non-fills, trapped air bubbles, loss of detail reproduction and difficult break away [10, 11]. These things did not happen. Why?
There are two possible reasons. One is the fact that a premium investment powder was used. Possibly this investment is fairly robust and can withstand some abuse. The second reason is the fact that a very simple and small pattern was used that probably did not challenge the mold enough, Figure 13.
To check the plausibility of the argument about using the premium investment, the investing and casting was repeated with a non-premium investment. The same simple pattern was used. The following are the observations.
There were severe watermarks at the higher water-to-powder ratios, Figure 14. Air bubbles were present at every water-to- powder ratio and to about the same degree. Changing the investing process reduced some of these problems slightly. However, the point is that problems arose, as the industry experts and investment manufacturers say they would.
It appears as though the question as to why the defects did not occur is answered. It did have something to do with using a premium investment. Perhaps if the pattern was larger and more difficult, even the premium investment would get poor results. Even though some good results were obtained at water-to- powder ratios outside the recommended range, do not be fooled. These results simply show that it is sometimes possible to abuse some investments without getting defects. The results are far from saying that no defects occur while using water-to-powder ratios outside the recommended range every time, in any environment or with any investment.
The data presented shows that there are several investment properties that are affected by water-to-powder ratio. As the water-to-powder ratio goes up, so does the pour time, set time and fluidity. The strength, however, goes down.
Within the recommended water-to-powder ratio range, the investment manufacturer has built in some flexibility to change these properties to the investor's liking. But, by going outside the recommended water-to-powder ratio range, casting defects can arise, especially with non-premium investments. It is, therefore, very important to monitor the water-to-powder ratio. Make sure that the recommended range is being used and measure the water and powder carefully.
Summary
Water quality is an important factor that affects the investment properties. So, the recommendation is to de-ionize the water before using. Changes in temperature can change the setting rate. So, the recommendation is to control the temperature to reduce variability. The water-to-powder ratio affects many investment properties. So, again, it is sensible to use a ratio within the recommended water-to- powder ratio range. Finally, note that the investment manufacturer has considerable knowledge and facilities. So use the technical support offered by the investment manufacturer. Using these principles to your advantage, you can make very intricate castings like the one in Figures 15 &16 made by Mike Kelley, the Jewelry Technical Specialist of Ransom & Randolph.
References
- Ralph Carter, "Effect of water quality and temperature on investment casting powders " ,Proc. of the Santa Fe Symposium on Jewelry Manufacturing Technology 2000, Met-Chem Research,2000, p 1-27. Also: Gold Technology, no 32,Summer 2001 ,p 7-18.
- Ralph Carter, "Effects of changing water-to-powder ratio on jewelry investments " ,Proc. of the Santa Fe Symposium on Jewelry Manufacturing Technology 2001," Met-Chem Research, 2001, p 31-47.
- Ceramic Industry 1999 Materials Handbook, January 1999, Business News Publishing Co. ,p 149-150.
- K. W. Witten, K.D.Gaily, R. E .Davis, General Chemistry, 3rd ed., Saunders College Publishing, 1988,p 468-472, 479- 480.
- C.W.Fetter,Applied Hydrogeology,3rd ed.,Macmillan College Publishing Co.,Inc.,1994,p 420-421.
- Ralph W. Phillips, Skinner's Science of Dental Materials, 9th ed., W.B.Saunders Co., 1991 ,p 70-78 & 393-411
- Ransom & Randolph, ISO 9002, Tier II Quality Procedures.
- "Application Instructions Ultra-Vest ®Jewelry Investment," Ransom & Randolph, July 1999.
- Omega,The pH and Conductivity Handbook,Vol.29,p 2-22.
- Dieter Ott ,"Properties and testing of investment ", Proc. of the Santa Fe Symposium on Jewelry Manufacturing Technology 1988," Met-Chem Research Inc.,1989,p 47-62.
- "Ransom & Randolph Setting Jewelry Standards Worldwide," Ransom & Randolph, section on Casting Defects: Potential Causes and section on Questions & Answers.
- Carl H. Schwartz," Chemical and physical properties of investment ", Proc. of the Santa Fe Symposium on Jewelry Manufacturing Technology 1987," Met-Chem Research, 1988,p 99-105.
- John A. Dean," Lange's Handbook Of Chemistry," McGraw- Hill,Inc., 14th edition 1992,p 5-87.
- Donald J. Petersen, Norbert W. Kaleta, Larry W. Kingston, "Encyclopedia of Chemical Technology," Kirk-Othmer,4th ed., vol.4, p 813.
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.