Enamel Preparation Method
The traditional method of enamel preparation was described by Cunynghame and Chapin. Large chunks of enamel were wrapped in a piece of cloth and broken with a hammer. The cloth was to prevent the particles from flying about, getting into one's eye or being lost. When the enamel had been broken into pieces about the size of a pea, they were washed to remove any lint.
13 Minute Read
The traditional method of enamel preparation was described by Cunynghame1 and Chapin2. Large chunks of enamel were wrapped in a piece of cloth and broken with a hammer. The cloth was to prevent the particles from flying about, getting into one's eye or being lost. When the enamel had been broken into pieces about the size of a pea, they were washed to remove any lint.
Quoting Cunynghame verbatim: "The enamel is placed in a very hard mortar, about 8 inches in diameter, preferably of Scottish or Villon granite, with a pestle of the same material. A little clean water is poured on to it, to prevent the chips from flying, and then it is pounded into small pieces with the aid of the mallet. The mortar may be laid on a bag of sand to prevent its being broken by the shock. Afterwards the enamel is ground up with the pestle to the size of ordinary sea sand."
Two paragraphs later: "After the enamel begins to become as small as sand, a milky substance seems to be disengaged and to fill the water, which lies above the enamel. This consists of some of the colouring matter of very fine particles of enamel and of potash and soda. If any of it is left in, the enamel when fired will be opaque and dull. Hence it must be washed away by agitating the pounded enamel in water poured into the mortar and then pouring off the fluid. This must be done until the enamel remaining is in fine even grains, looking like perfectly clear, clean, fine sand. The size of the grains may be such as will go through a fine sieve with meshes 75 to the linear inch."
Five paragraphs later: "Opaque enamels need not be washed, except to remove any little dirt that may have got in, and, as will presently be seen, some coatings of enamel cannot be washed, but must be put on in a state of impalpable powder. So thin, however, are the layers thus used that they are fused up into transparent enamel." As far as we can determine, he did not expand on this statement presently or later. If he had, he probably would have said that low firing enamels made at this time were practically water soluble.
Unfortunately, the art-enameling community has always been isolated from the glass and enamel scientists. As a result, Cunynghame cannot be criticized, because he did not have the benefit of knowledge gained during the eighty years following publication of his book.
During the last decade of the nineteenth century, glass scientists became very involved with the durability of glass. The first published work of note was by Foerster3 in 1893. Much has been published up to the present. A few of the major works are listed as references.
Let us start with the enamel as it is removed from the pot and poured onto a metal plate to cool. At this stage, it is a round flat disk perhaps eight inches in diameter and one half inch thick. Depending on the composition, some are quite durable as to water, acid and alkali. Others are not so durable. In any case, they all are at their maximum durability at this moment. Surface tension caused the glass to assume a minimum volume, thus tightening the network structure at the surface, forming what we might call a fire polished surface. Any free alkali at or near the surface is vaporized, resulting in a skin which is a little more durable than the interior.
When cool, the enamel cakes are ground or crushed. The normal European method is ball milling, while the normal method in the United States is crushing with hardened steel rolls. The principle advantage of the latter method is fewer fines are produced.
Ground enamel is slightly less durable than the cake or lump form. When two immiscible phases, such as a gas and a solid are brought into contact, the solid will adsorb a thin film of the gas.
Adsorption is to be distinguished from absorption, which involves the bulk penetration of the structure of a solid by a gas and is governed by laws of diffusion.
Air is a gas which normally contains some water. Thus, at certain humidity and temperature conditions, all solid surfaces will adsorb a thin film of water. A fire polished surface will adsorb only a thin layer, known as physical adsorption. Such layers are weakly bonded and can be removed by a slight increase in the temperature of the solid.
When glass is broken, an atomically clean surface is exposed. Immediately, this clean surface reacts with the air by a process known as chemisorption. If the certain humidity and temperature conditions mentioned above exist, the surface will adsorb a thin film of water. Unlike physical adsorption, chemisorption consists of strong bonds and the water cannot be removed by a slight increase in the temperature of the glass.
The thin film of water reacts with the glass. First is an ion exchange of alkali and hydrogen ions (explained below), and second, the formation of sodium hydroxide and/or sodium carbonate. If the humidity and temperature conditions change, the sodium hydroxide and/or sodium carbonate may crystallize and cease to react. If conditions change so the crystals can absorb water, reaction will restart.
The extent of the reaction depends upon the composition of the enamel, and the precautions exercised by the manufacturer, distributor, and enameler.
The scene now switches to the enameler's studio. According to most books, all enamels are immediately washed and stored wet, in small jars or bottles.
At least three different steps are involved in the reaction of water with ground enamel. The first, is ion exchange of hydronium (H3O+) or hydrogen ions from the water with alkali ions in the glass. Second, is the partial hydration of the silicon-oxygen network of the glass. Third, is the dissolution of the glass into the contacting solution.
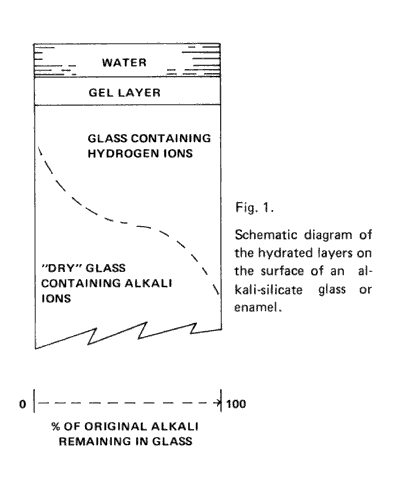
Figure 1 is a schematic diagram showing water in contact with one surface of glass, assuming the other surfaces are protected. The dry glass at the bottom, contains alkali ions at the original concentration. As one proceeds upward to the glass surface, there is a decrease in the concentration of alkali ions (dotted S curve) as a result of their replacement with hydronium ions. In this layer of partial exchange, the network structure of the glass is intact, and the only change is replacement of one ion for another. Closer to the surface, the network can become partially hydrated by reaction of silicon-oxygen bonds with water.
This partial hydration leads to a more open structure than in the original glass; ions from solution and water molecules can penetrate through this partially hydrated or gel layer with mobilities much higher than in the glass network that has not been broken up by reaction. (1)
The exchange of alkali ions in the glass and hydronium ions from water can be described with the equation: Na+ (glass) + H3O+ (solution) = Na+ (solution) + H3O+ (glass). (2)
As reaction (2) proceeds the solution becomes more basic, and the rate of dissolution of the silicon-oxygen network becomes more rapid.
At extended time of reaction, the amount of alkali appearing in solution becomes proportional of time. Furthermore, silicon and other glass constituents are found in the solution. These results suggest that the glass dissolves into the solution by reactions of the type:
- 2 H2O + SiO2 = H4SiO4 (3)
- H2O + CaO = Ca(OH)2 (4)
- 3 H2O + A12O3 = 2A1(OH)3 (5)
- H2O + Na2O = 2 Na(OH) (6)
- H2O + PbO = Pb(OH)2 (7)
In reaction (2), the sodium cation (ion with a positive charge) was used as an illustration because it has the greatest mobility in a glass network. Actually, all cations react with the hydrogen ion as shown in reaction (2), but at different rates.
The rate of the above reactions depend greatly on the composition of the enamel and to some extent on the amount of water used, as well as the temperatures of storage, and whether or not the jar is tightly sealed. When a sufficient amount of the enamel has been dissolved, the mass will 'set up' like concrete. Enamels have been made with durability so low that they would 'set up' in a matter of days. Enamels can also be made with durability so high that it takes years.
Since the attack is proportional to time, it is obvious that fine particles would be completely changed to a gel before large particles. The composition of the gel will vary some, depending on the composition of the enamel. In any event, it is composed of metal hydrates which will not form a glass at a normal firing temperature. Therefore, it is desirable to remove the fine particles which have a high degree of deterioration. The custom has been by elutriation as described by Cunynghame. This does not remove the gel or deterioration from the larger grains, which may give off water up to 1000°F or higher, leaving metal salts which will not be taken into solution by the glass at normal firing temperatures, resulting in white specks.
If some combination of acids and/or alkali could be used to completely dissolve the gel, there remains that portion of the glass where there is a partial exchange of hydrogen ions for alkali ions. Once most of the hydrogen ions and water molecules have been driven out with heat, there remains a silica rich area which is more refractory and of a much lower expansion than the bulk of the glass. This part of the glass will have a dull appearance and some opacity due to some water being retained. Perhaps these silica rich areas can be removed with hydrofluoric acid, but how do you stop just short of dissolving too much silica and end up with surfaces too rich in alkali?
There is an alternate method to elutriation. It is called screen separation. Three screens, 100 mesh, 200 mesh, and 325 mesh should be sufficient for most purposes. Enamel classified through 100 mesh and remaining on 200 mesh is ideal for good transparents. That which passed through the 200 and remained on 325 can be ground in a mortar and pestle to pass through 325 and used for painting. Normally, opaque enamels can be used without screening out the fines. In rare cases, it might be helpful to remove particles finer than 325 mesh.
Figure 2 shows a set of two screens along with a collecting pan and a cover. Note a couple sizeable lumps of frit in the screen will aid in keeping the wire cleared to speed up the process.
Figure 3 shows how the screens nest making the operation easier. Of course, the enamel can be screened in single screens without nesting.
No doubt some enamelers will feel some extremely fine powder will adhere to the grain surfaces throughout the screening and wish to remove it by washing. Alcohol is ideal for washing enamel. It has high affinity for water and evaporates readily. Ethanol (ethyl or grain) should be used, even though it is more expensive. Methanol (Methyl or wood) is poisonous if taken internally or with prolonged breathing of the fumes.
Although we have pointed out water can be a source of problems with some enamels, others are quite resistant to water and little or no problem will develop. Our concern is the teaching of washing as a fundamental principle. It should be taught as a special operation for a special purpose, if taught at all.
If we were determined to use certain enamels and were concerned with obtaining transparency, we would purchase it in lump form, and grind it in a mortar with a transparent plastic cover with a hole in the center to allow the handle of the pestle to stick through. We would grind a short time, screen, regrind, screen, continuing until enough enamel of the proper mesh was obtained. We would wash, only if necessary, with water or alcohol. Any left over enamel that had been exposed to water, should be discarded. Any ground enamel, which has not been exposed to water, should be stored in a desiccator. Again, it is stressed, all enamels do not require this degree of pampering.
Enamel being attacked by water is not a unique phenomenon. Water attacks all glass, especially when freshly broken or ground into a powder. An enlightening experiment is to place a piece of window glass in distilled water and ad a few drops of phenolphthalein. No reaction will be indicated. Grind the piece of glass into a powder, add water and a few drops of phenolphthalein. The solution will immediately turn pink, indicating the presence of alkali in the solution.
Of historical interest, are the following two excerpts: The first from Cellini4, written 1568: "We have a proverb in the craft which says, 'Smalto sottile e niello grosse.' 'Enamel should be fine, niello should be coarse', and that's just what it is. You put your enamel in a little round mortar of well-hardened steel, and about the size of your palm and then you pound it up with very clean water and with a little steel pestle especially made for this purpose of the necessary size. Some, to be sure, have pounded their enamels on porphyry or serpentine stone, which are very hard and more over have done this dry, but I now think that the steel mortar is much better, because you can pound it so much cleaner."
The second excerpt is from the third edition (published in 1906) of Cunynghame, page 91:
"But, since this edition was published, Mr. Charles Tomes, F.R.S., has made some interesting experiments which shed new light upon the subject." They will be found in the August number, 1900, of the Journal of the Society of Arts. His conclusion is, "that the apparent mud only consists of finer particles of the very same composition as the coarser stuff, and that these fine particles, especially on the surface, become very quickly agglutinated by the heat of the furnace, entangling between and beneath them an infinity of small bubbles but that, when coarser particles are fired, they run together more slowly, and then the air escapes for the most part, the little which remains forming large bubbles, which do not practically interfere with the transparency."
"The experiments of Mr. Tomes undoubtedly bear out the general proposition put forward by him. He concludes that grinding enamels in paraffin oil* is not better than to grind them in water." *(In the U.S., paraffin oil is called kerosene.)
"In this as a practical result, I am unable to agree with him, for although enamels kept under water suffer but little change, enamels kept for many days in a state of fine, damp, mud undoubtedly appear to undergo decomposition."
The observations of both Tomes and Cunynghame were correct. If Tomes applied the fine enamel immediately after grinding, the small bubbles would have been the only difference. And, had he applied the enamel thin, he might have eliminated most of the small bubbles. Cunynghame was correct in that small particles deteriorate faster than large particles. Small particles have more surface area in proportion to their volume than do larger particles.
When working with transparent enamels, one of Cunynghame's remarks quoted earlier in this article should be considered a fundamental principle: "So thin, however, are the layers thus used, that they are fused up into transparent enamel."
We hope those who experiment using alcohol for washing will share their experience with Glass on Metal Magazine.
References
- Cunynghame, Henry, On the Theory and Practice of Art-Enamelling Upon Metal . Archibald Constable & Company, Westminster. 1899. Pp. 48-51
- Chapin, Howard. How To Enamel. John Wiley & Sons, New York. 1911. Pp. 1-7.
- Foerster, F.Z. Instrumentenk . Vol. 13, pg. 457. 1895.
- Ashbee, C.R. The Treatises of Benvenuto Cellini on Goldsmithing and Sculpture Dover Publications, Inc., New York. 1967. Pg. 17.
- Pye, L.D. & others. Introduction to Glass Science, Plenum Press, New York. 1972. Pp. 513-529. Tomozana, M. & others.
- Treatise on Materials Science and Technology Vol. 17. Academic Press, New York. 1979. Pp. 41-69. Doremus, R.H.
- Glass Science John Wiley & Sons, New York. 1973. Pp. 213-228. Scholes, S.R
- Modern Glass Practice Industrial Publications Inc., Chicago. 1951. Pp. 262-266 Shand, E.B
- Glass Engineering Handbook. McGraw-Hill Book Company, Inc., New York. 1958. Pp. 91-102
- Wang, F. & Tooley, F.V. "Detection of Reaction Products Between Water and Soda-Lime-Silica Glass", Journal American Cer. Soc., Vol. 41. 1958. Pp. 467-469, 521-524
- Simpson, H.E. "Measuring Surface Durability of Glass", Ceramic Bulletin, Vol. 30. 1951. Pp. 41-45
- Eppler, R.A. & others. "Resistance of Porcelain Enamels to Attack by Aqueous Media," Ceramics Bulletin, Vol. 56. 1977. Pp. 1064-1070. Ceramic
- Bulletin Vol. 60. 1981. Pp. 618-622.
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.